Most of the 38 million people living with diabetes In the U.S., daily injections or insulin pumps are used to keep glucose at safe levels, but new research suggests a third option could be just as effective.
In a study led by Irl B. Hirsch, MD, medical director of the Center for Diabetes Care at the University of Washington Medical Center, a inhaled form of insulin (similar to an asthma inhaler) worked as well as injections or pumps to control type 1 diabetes.
The research was presented last week at the 84th Scientific Session of the American Diabetes Association (ADA) in Orlando, Florida.
EATING YOGURT COULD HELP PREVENT A COMMON DISEASE, ACCORDING TO THE FDA
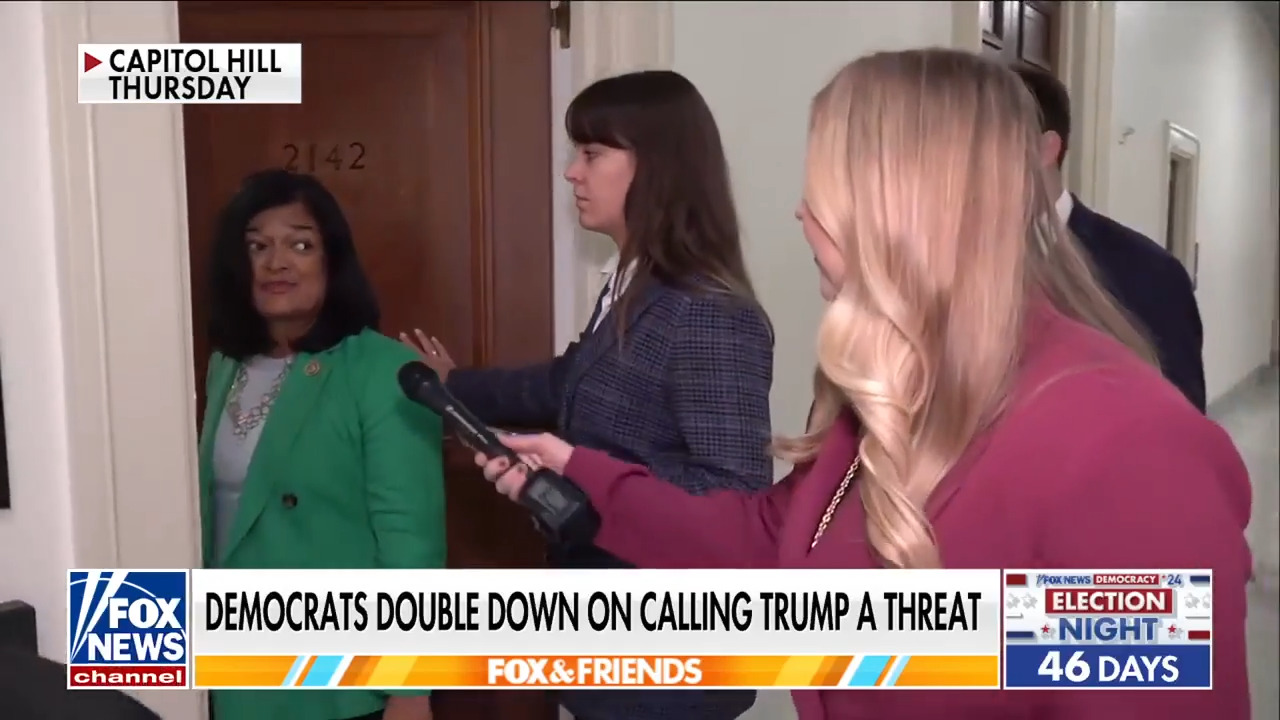
The clinical trial tested a product called Afrezza, an inhaled basal insulin manufactured by MannKind Corporation in California.
Afrezza, the only inhaled insulin on the market, has been available since gaining FDA approval in June 2014.
In a recent study, an inhaled form of insulin worked as well as injections or pumps in controlling type 1 diabetes. (iStock/MannKind)
Benefits of a third option
“In those with type 1 diabetes, insulin is required for survival“Hirsch told Fox News Digital in an interview.
“With continuous glucose sensing, glucose control has improved dramatically, but not everyone hits the target with multiple injections or pumps, and each therapy has many pros and cons,” he said.
EATING ONE TYPE OF FRUIT REGULARLY COULD REDUCE THE RISK OF DIABETES IN WOMEN, STUDY SUGGESTS: 'INCREDIBLY HEALTHY'
With pumps, people must wear the device, which can cause skin problems.
They also have to buy additional accessories.
Blood glucose levels can also fall with exerciseHirsch warned, which can be problematic.

Afrezza, an inhaled basal insulin shown here, is manufactured by MannKind Corporation in California. (MannKind)
“Injections in general may be more convenient for some, but they don't work as well as pump patients,” he said.
With Afrezza, the product is inhaled into the lungs before meals, and the rapid-acting insulin minimizes the glucose spike often seen after eating, Hirsch noted.
“Patients with type 1 diabetes should consider this as another option for their mealtime insulin and talk to their doctor about this option.”
During the 17 week studyResearchers evaluated the outcomes of 141 adults who were assigned to use the Afrezza inhaler or continue with traditional injection or pump delivery methods.
At 17 weeks, all participants switched to the inhaler for another 13 weeks.

Dr. Irl B. Hirsch, MD, medical director of the Center for Diabetes Care at the University of Washington Medical Center, led the new study. (MannKind)
All groups were assessed with continuous glucose monitoring at baseline, at 17 weeks, and again at 30 weeks.
Among the inhaled insulin group, 30% of participants reached their glucose target levels (less than 7% blood sugar) compared to 17% of people using injections and pumps.
There were no differences in hypoglycemia (low blood sugar) between the groups.
UTAH MOM FIGHTS FOR HER DAUGHTER'S ACCESS TO DISCONTINUED DIABETES MEDICATIONS: 'SAVES LIVES'
“Overall, there were no differences in our primary endpoint, HbA1c, a reflection of average blood sugar level,” Hirsch said.
“But that alone is misleading: Many patients did better in glucose control, while others did worse.”
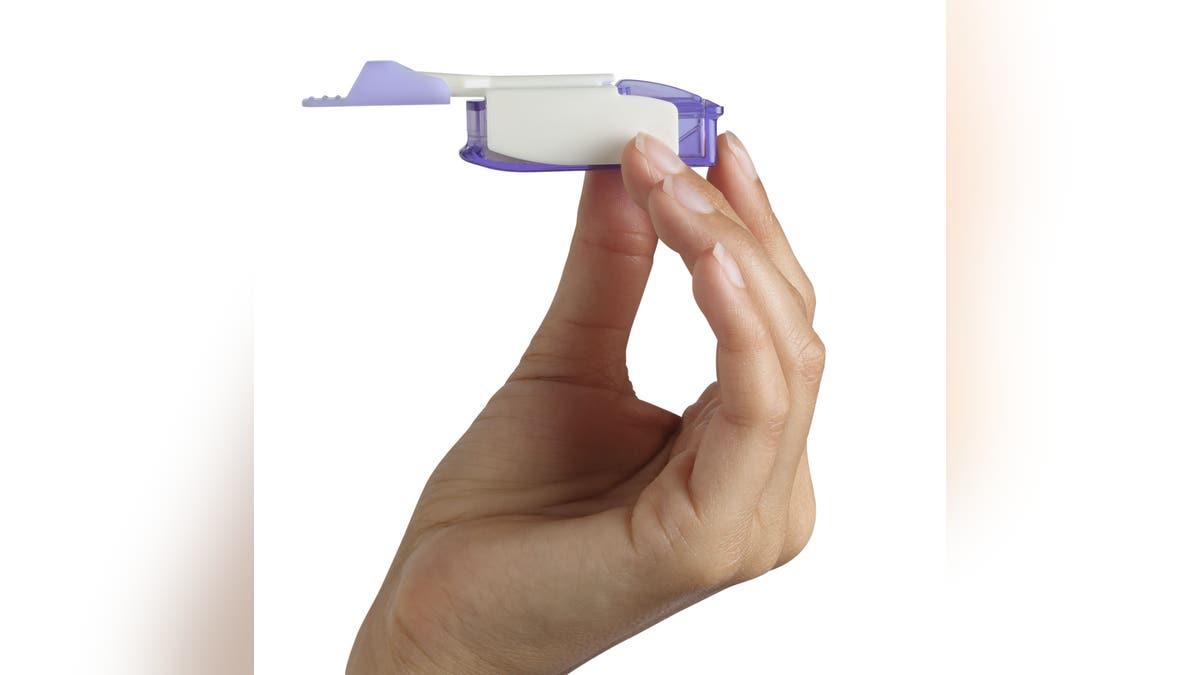
With Afrezza, the product is inhaled into the lungs before meals, and the rapid-acting insulin minimizes the glucose spike often seen after eating, one doctor said. (MannKind)
“The thing is, inhaling insulin isn't for everyone, but some people did better than with their pumps.”
The people who did best inhaled insulin. between meals and at bedtime, Hirsch added.
CLICK HERE TO GET THE FOX NEWS APP
At the end of the study, more than half of the participants said they would choose to continue inhaled insulin therapy.
“The most important takeaway is that patients with type 1 diabetes should consider this option as another option for their mealtime insulin, and talk to your doctor about this election,” he recommended.
'Adds value'
The American Diabetes Association acknowledged the promise of the study's findings in an email to Fox News Digital.
“We look forward to our scientific sessions each year to see data like the INHALE-3 study findings, which have the potential to expand diabetes care“Raveendhara Bannuru, MD, PhD, vice president of medical affairs and quality improvement outcomes at the ADA in Boston, Massachusetts, told Fox News Digital via email.

“With continuous glucose sensing, glucose control has improved dramatically,” one doctor told Fox News Digital. (iStock)
“We are hopeful for the continued development of alternative insulin delivery methods that could offer options for people living with diabetes,” the group also said in the statement.
“The INHALE-3 trial demonstrated that inhaled insulin, combined with insulin degludec, effectively reduces A1c levels without increasing hypoglycemia or weight gain in people with type 1 diabetes. This adds value to options in insulin therapy “.
Potential risks and limitations
While more people met their glycemic goals with Afrezza, some subjects saw worse readings when switching from usual methods to inhaled insulin, “potentially due to missed doses of inhaled insulin during the day and/or insufficient dosing before bedtime.” “the researchers wrote.
CLICK HERE TO SUBSCRIBE TO OUR HEALTH NEWSLETTER
“We didn't see any concerns,” Hirsch said when asked about side effects.
“As expected, some people coughed immediately when dosing their insulin, but no major concerns were noted and everyone continued on their inhaled insulin.”
“Not everyone hits the target with multiple injections or pumps, and each therapy has many pros and cons,” said one doctor. (iStock)
The most common side effects seen in the study were hypoglycemia, cough, and sore or irritated throat.
Afrezza has been linked to a risk of acute bronchospasm in patients with chronic lung disease, such as asthma or COPDaccording to the manufacturer.
“Insulin inhalation is not for everyone, but some did better than with their pumps.”
Before starting Afrezza, patients should see a doctor for a physical examination and tests to measure pulmonary function.
Patients who smoke or have recently quit smoking should not take the inhaled medication.
For more health articles, visit www.foxnews/health
Fox News Digital reached out to MannKind for additional comment.