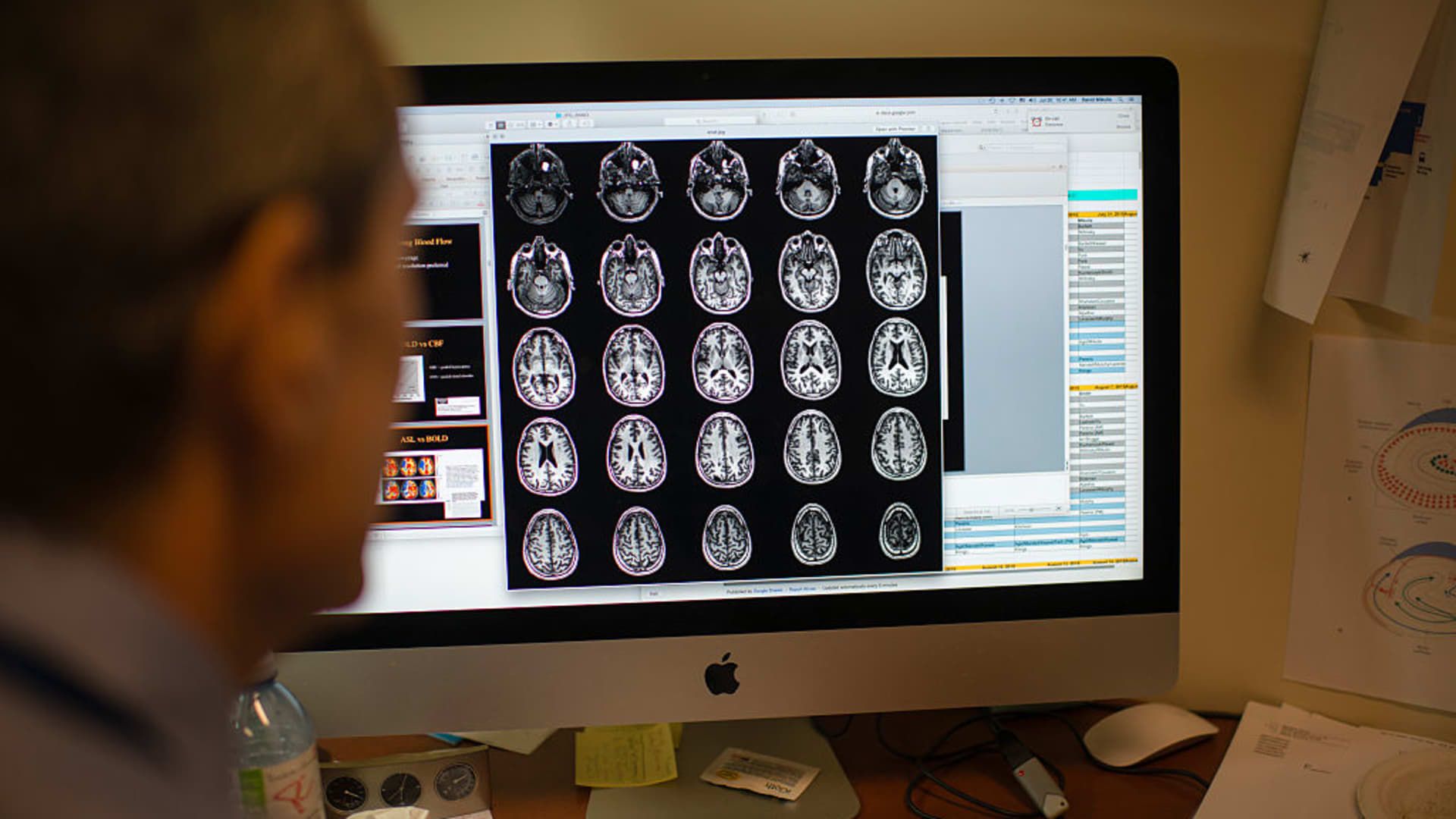
Club holding company Eli Lilly hopes to win approval for its Alzheimer's treatment in the coming weeks, but investors looking for immediate financial success should temper their expectations. On its fourth-quarter earnings call Tuesday, rival pharmaceutical maker Biogen shared data showing its therapy for the memory-robbing disease Leqembi is off to a slower-than-expected start. Bottom line: Acceptance of the drug, the first of its kind to receive full regulatory approval in the U.S., has been limited by obstacles in the health system, including access to dementia specialists who can make an Alzheimer's diagnosis . Eli Lilly's experimental treatment, which is similar to Leqembi in the way it attacks the disease and is administered to patients, is likely to face the same hurdles if approved by the Food and Drug Administration, despite its promise long-term. The FDA's decision on Lilly's drug, known as donanemab, is expected in late March. Biogen's latest update underscores the challenges Lilly faces in making donanemab a commercial success. Meanwhile, Eli Lilly's diabetes and obesity drugs, the heart of our investment thesis, should continue to drive the majority of the company's top-line growth. About 2,000 patients are currently taking Leqembi, Biogen said Tuesday, up from 800 at the time of the company's third-quarter report in November. Despite the progress, Biogen and its drug partner, Japan's Eisai, are unlikely to reach their previous goal of 10,000 Leqembi patients by the end of March. The Food and Drug Administration granted full approval to Leqembi in July, a landmark decision that allowed the U.S. government's senior health insurance plan, Medicare, to provide reimbursement for the treatment. “We are clearly seeing that there is demand for the product,” Biogen CEO Chris Viehbacher said Tuesday. However, the challenge is converting people who want to receive Leqembi into beneficiaries due to bottlenecks in the health system. In addition to the number of dementia specialists, Viehbacher said scheduling a follow-up MRI to monitor the medication's side effects has also prevented eligible people from starting treatment. About 3,800 people are on an Alzheimer's Association registry for Leqembi recipients or people close to starting treatment, Viehbacher said. That means between 260 and 265 people are being added to the registry each week, about 56% more than in December, Viehbacher said. The registry data cited by Biogen is “encouraging, but we expect conversion to therapy,” Morgan Stanley analysts said in a note to clients. Due to the slower-than-expected rollout, analysts lowered their 2024 Leqembi revenue estimates to $370 million from $470 million, while 2025 estimates were revised more modestly to $1.1 billion from 1.2 billion dollars. Still, Morgan Stanley reiterated its equivalent buy rating on Biogen shares, citing optimism that Leqembi adoption will reach an inflection point this year, even if the exact timing is uncertain. Approval of Lilly's donanemab therapy for Alzheimer's could come any day. The Indianapolis-based pharmaceutical giant reiterated last week that it expects a decision from the FDA in the first quarter, which ends March 31. The decision could have implications for the Club's holding company GE Healthcare, which makes MRI machines used to monitor side effects. We expect the medical equipment maker to benefit from the launch of Alzheimer's drugs in multiple ways, including increased demand for its MRI machines and Vizamyl, a tracking agent used to measure amyloid plaque and aid in the diagnosis of Alzheimer's. patients. LLY YTD lifts Eli Lilly's stock performance so far in 2024. Donanemab, and Lilly's next-generation Alzheimer's therapies, should help drive the company's growth in the coming years. But in the short term, as the experience of Biogen and Eisai shows, investors should keep their donanemab sales expectations in check. Lilly's diabetes and obesity treatments, led by Mounjaro and Zepbound, remain the biggest drivers of its financials and stock, which have been a big winner in recent years, including so far in 2024. In many ways , the initial success of the drugs, which share the active ingredient tirzepatide, offers Lilly a respite from the long-awaited launch of donanemab. Jim Cramer has long said that tirzepatide could become the best-selling drug of all time. Wall Street projects that Eli Lilly will generate $41.07 billion in revenue in 2024, with donanemab contributing about 0.5%, or $185 million, of the company's total, according to estimates compiled by FactSet. Meanwhile, Mounjaro and Zepbound are expected to generate a combined $11.7 billion, FactSet data shows. Pharmaceutical companies, including Biogen and Eli Lilly, have spent billions of dollars over the years developing experimental treatments for Alzheimer's, but Leqembi is the first drug designed to slow the progression of the disease to receive the traditional FDA clearance. Thus, Biogen and Eisai are the first companies to encounter bottlenecks in the health system for the treatment of Alzheimer's, although analysts believe that Eli Lilly's expected presence in the market will help alleviate some of the challenges, benefiting the adoption of both Leqembi and donanemab. Both drugs are antibodies that seek to eliminate the abnormal buildup of a protein called amyloid in the brain, based on the belief that doing so can slow the progression of Alzheimer's. Clusters of amyloid, usually called plaques, have long been associated with Alzheimer's, although their exact role in the disease is not fully understood. Side effects of anti-amyloid drugs include swelling of the brain and bleeding. In an 18-month late-stage study, donanemab slowed cognitive and functional decline by 35% in a group of early Alzheimer's patients compared to people taking a placebo. Leqembi slowed disease progression by 27% in its 18-month trial, but the drug is generally considered to have a better safety profile than Eli Lilly's therapy. Leqembi is administered intravenously every two weeks, while donanemab doses are received intravenously every four weeks. Leqembi side effects are monitored by regular MRI scans during treatment. According to the FDA label, patients need an initial MRI before receiving the drug and then additional scans before the fifth, seventh, and fourteenth infusion. If the FDA approves Lilly's donanemab, a key focus will be whether it has different screening and monitoring requirements compared to Leqembi, Morgan Stanley analysts also said in the note. (Jim Cramer's Charitable Trust is long LLY and GEHC. See here for a full list of stocks.) As a subscriber to the CNBC Investing Club with Jim Cramer, you will receive a trade alert before Jim makes a trade. Jim waits 45 minutes after sending a trade alert before buying or selling a stock in his charitable fund's portfolio. If Jim has talked about a stock on CNBC TV, he waits 72 hours after issuing the trade alert before executing the trade. THE ABOVE INVESTMENT CLUB INFORMATION IS SUBJECT TO OUR TERMS AND CONDITIONS AND PRIVACY POLICY, TOGETHER WITH OUR DISCLAIMER. NO FIDUCIARY OBLIGATION OR DUTY EXISTS OR IS CREATED BY VIRTUE OF THE RECEIPT OF ANY INFORMATION PROVIDED IN RELATION TO THE INVESTMENT CLUB. NO SPECIFIC RESULTS OR BENEFITS ARE GUARANTEED.