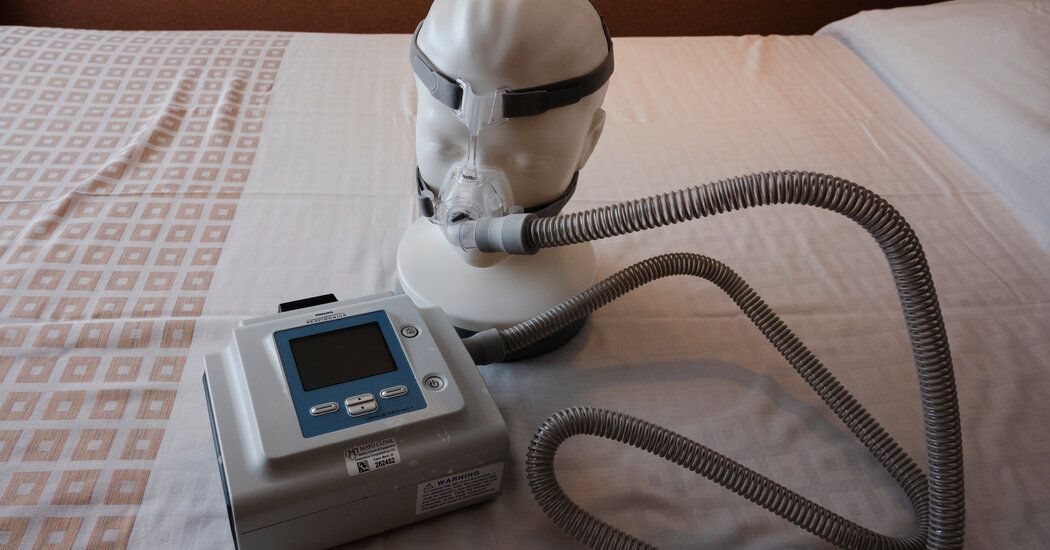
Philips Respironics announced Monday that it would stop sales of all of its ventilators in the United States after reaching a settlement with the Food and Drug Administration over ongoing problems with the devices.
Millions of the company's ventilators and CPAP machines, used to facilitate breathing at night, were recalled after reports that they spewed chunks of foam and potentially toxic gases into consumers' airways.
Under the agreement, Philips said it would have to meet a list of standards in a “multi-year” plan before it could resume business in the United States. The company said more details would be revealed when the settlement was finalized in court. But it added that it would continue to repair existing devices and provide service to the people who use them.
The company initially began recalling millions of devices in June 2021 and halted sales of new sleep therapy machines in the United States, according to Philips spokesman Steve Klink. At the time, the company and the FDA cited the potential for serious injury or permanent impairment due to potentially carcinogenic chemicals emitted by the devices.
The company has since released additional test results, saying the devices “were not expected to cause measurable harm to patients' health” and said it would continue testing. The FDA has rejected some of the company's updated claims, at one point calling them “unconvincing.” Philips has also faced continued scrutiny and issued more recalls in its attempts to update devices.
Dr. Jeff Shuren, director of the FDA's devices division, said the agency could not comment until the settlement was finalized and filed in court.
The initial recall affected about 15 million respirators produced since 2006, although approximately five million were still in circulation as of mid-2021.
Since replacements were not immediately available, the recall caused confusion and upset for many doctors and patients. Many struggled to weigh the risk of continuing to use a faulty device against the danger of sleeping with breathing problems.
Millions of people suffer from sleep apnea, or interrupted breathing, which is associated with high rates of strokes, heart attacks, and possible cognitive decline. The recalled machines included CPAP, or continuous positive airway pressure, machines; BiPap devices; and fans.
Amsterdam-based Philips revealed it had reached a negotiated settlement, or consent decree, with the U.S. Department of Justice and the FDA, along with announcing its fourth-quarter earnings. The company said it wrote off around 363 million euros related to the cost of completing the requirements of the agreement. Its shares, listed in the United States, fell about 7 percent on Monday morning.
The company said it would continue to sell its products in other countries.
Thousands of patients have since sued Philips, alleging that the machines caused a wide range of respiratory and other ailments, including allegations of deaths from lung cancer. In September, the company reached a $479 million settlement with plaintiffs to cover financial losses related to repairing or replacing the machines. Litigation over illnesses and medical costs are still pending.