David Ricks, CEO of Eli Lilly
Scott Mlyn | CNBC
Eli Lilly It expects its experimental weight-loss pill to be approved early next year, Chief Executive David Ricks told Bloomberg TV on Monday.
The company will release key data from the late-stage trial on the drug, or forglipron, later this year.
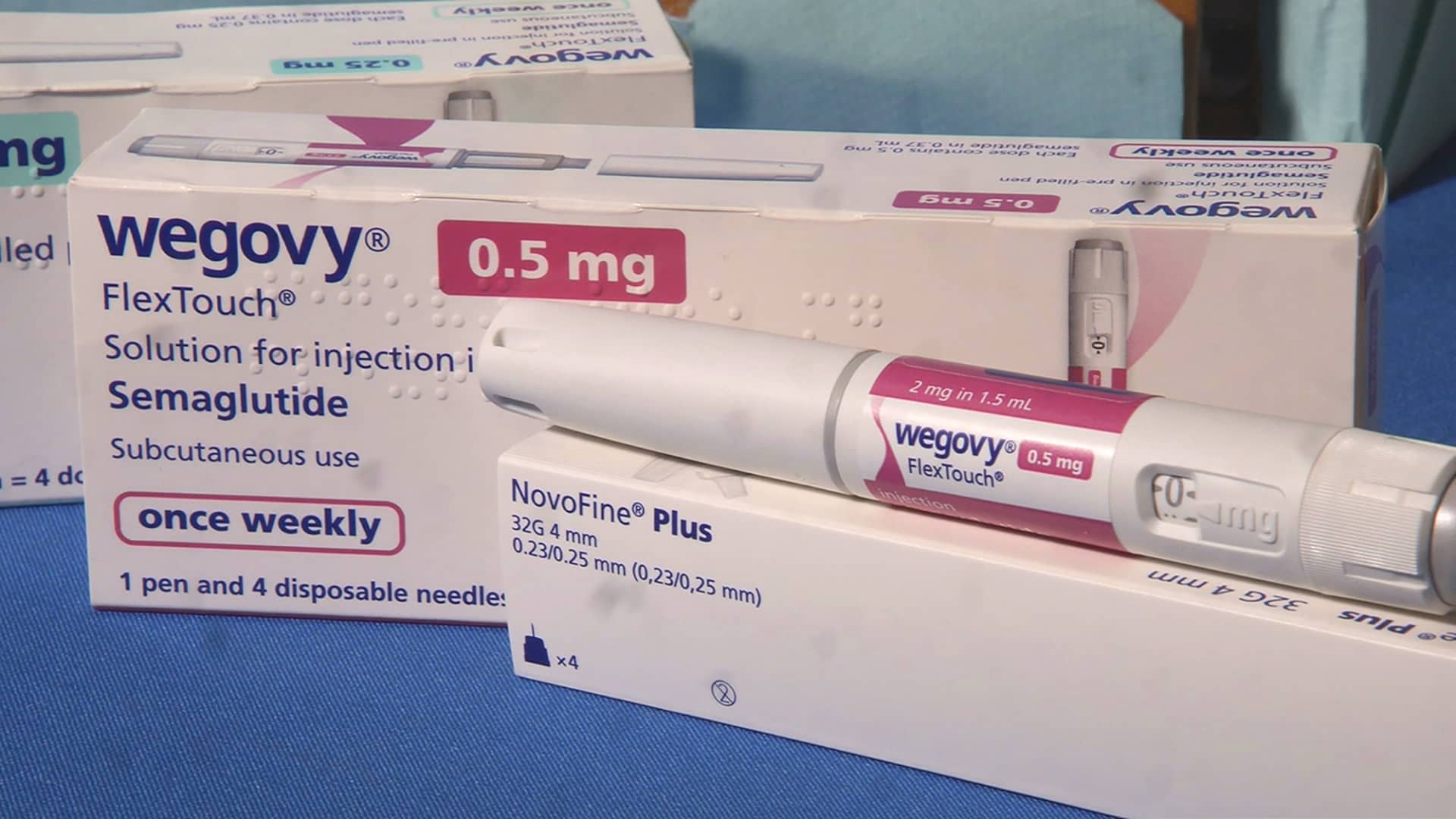
Eli Lilly is pushing to bring the pill to market as it competes with Nordisk and smaller rivals for a major share of the booming weight-loss drug market. Eli Lilly's Zepbound and Novo Nordisk's Wegovy dominate the space, but drugmakers and their competitors have been working to develop improved versions of the drugs.
The pills would be more convenient for patients than the current injectable forms. They would also be easier to manufacture at a time when Eli Lilly and Novo Nordisk have struggled to produce enough drugs to meet growing demand.
Eli Lilly has said that orforglipron helped patients lose up to 14.7% of their weight in a mid-stage trial, compared with 2.3% among people who took a placebo.
Eli Lilly shares fell slightly on Monday.