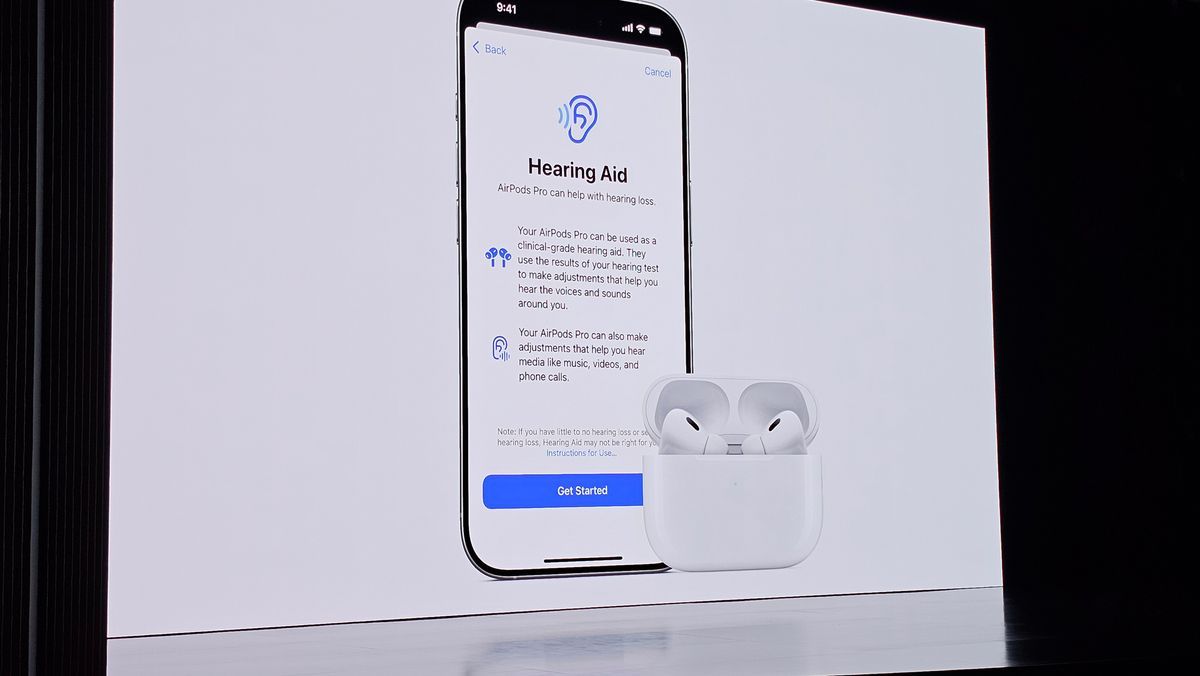
At Apple’s “It’s Glowtime” special event, the tech giant unveiled a pretty important and powerful health feature coming to the AirPods Pro 2. Of course, it’s the upcoming “Hearing Aid” feature along with hearing protection and a hearing test feature, all of which would arrive in a software update at no additional cost.
Now, three days after Apple revealed the feature and noted that it was pending FDA clearance, it has just been given the green light. The U.S. Food and Drug Administration (aka FDA) authorized AirPods Pro headphones are billed as the “first over-the-counter (OTC) hearing aid software device.”
This is a critical step for Apple, as they needed this approval to implement the feature. It is also a huge achievement for the over-the-counter hearing aid industry, and Apple getting the first ever approval is a significant step.
The acting director of the FDA’s Center for Devices and Radiological Health said, “Today’s marketing authorization of over-the-counter hearing aid software in a widely used consumer audio product is another step in promoting the availability, accessibility, and acceptability of hearing support for adults with mild to moderate perceived hearing loss.”
The FDA evaluated the feature “in a clinical study of 118 subjects with mild to moderate perceived hearing loss across multiple U.S. sites,” and the results “demonstrated that subjects using the HAF self-fitting strategy achieved similar perceived benefit as subjects receiving a professional fitting of the same device.” This validation by the FDA led to Apple’s general marketing authorization of the feature, which is likely consistent with the testing and studies the tech company conducted internally.
The addition of hearing aid functionality to AirPods Pro is a big step, especially for people with mild to moderate hearing loss. As an over-the-counter solution, and indeed a software update coming to existing AirPods Pro 2, it offers an alternative to purchasing other over-the-counter hearing aids, which can be more expensive.
Once available, to use the hearing aid feature on AirPods Pro 2, you'll need to take the Hearing Aid Test to establish a hearing profile, and then AirPods Pro 2 will make real-time adjustments to boost sound. Apple notes that AirPods Pro 2 will function like a “clinical-grade hearing aid,” and users will be able to make adjustments to tone, volume, and balance whenever they want.
Additionally, this FDA authorization allows Apple to implement the hearing test feature in the AirPods Pro 2, which TechRadar editor-at-large Lance Ulanoff had the chance to testThis feature, along with the hearing aid feature and other hearing protection built into AirPods Pro 2, takes the headphones beyond simply being a listening device to connect with other people. It builds on the noise level alerts for what you’re listening to on AirPods and the ambient noise alerts built into Apple Watch.
Now that Apple has received FDA clearance for its AirPods Pro hearing aid feature, we're likely one step closer to implementing it. As announced during the keynote and in a press release, Apple plans to launch the hearing test and hearing aid features this fall in more than 100 countries, including the United States, Germany, and Japan.
These will come in the form of a software update for AirPods Pro 2 alongside an iPhone or iPad running iOS 18 or iPadOS 18.