Celsopupo | Stock | fake images
Zealand Pharma outlined an ambitious five-year strategy for its anti-obesity portfolio on Thursday, highlighting how growing competition from smaller players is tightening the race for market leaders Novo Nordisk and Eli Lilly as more of these drugs get closer to entering the market.
The new strategy, “Metabolic Frontier 2030”, comes as Zeeland shares have fallen 29% so far this year as investors bet the market will fragment, with fewer singular winners than 18 months ago at the peak of the weight-loss drug frenzy.
Ahead of its Capital Markets Day on Thursday, the Danish drugmaker said it is now targeting five drug launches, at least 10 clinical programs in development and industry-leading cycle times by 2030.
The strategy will combine strategic partnerships, accelerated drug development and expanded research capabilities to build the world's most valuable metabolic health pipeline, Zealand Pharma said in a statement.
One of Zealand's most promising drugs in development is petrelintide, which targets the pancreatic hormone amylin, different from the gut hormone GLP-1 targeted by Novo's Wegovy and Lilly's Zepbound. The drug, developed in collaboration with Rochehas shown milder side effects than current injectables in early-stage clinical trials.
Mid-stage data on petrelintide will be delivered early next year, while data on its dual GLP-1 agonist called survodutide will be read throughout 2026.
Fewer notable winners
Novo Nordisk and Eli Lilly currently dominate the weight loss drug market and have an advantage over their competition, having developed the only anti-obesity drugs approved by the Food and Drug Administration to date. But as the market takes shape, more players want to participate in this lucrative business, which analysts say could be worth up to $150 billion annually by the beginning of the next decade.
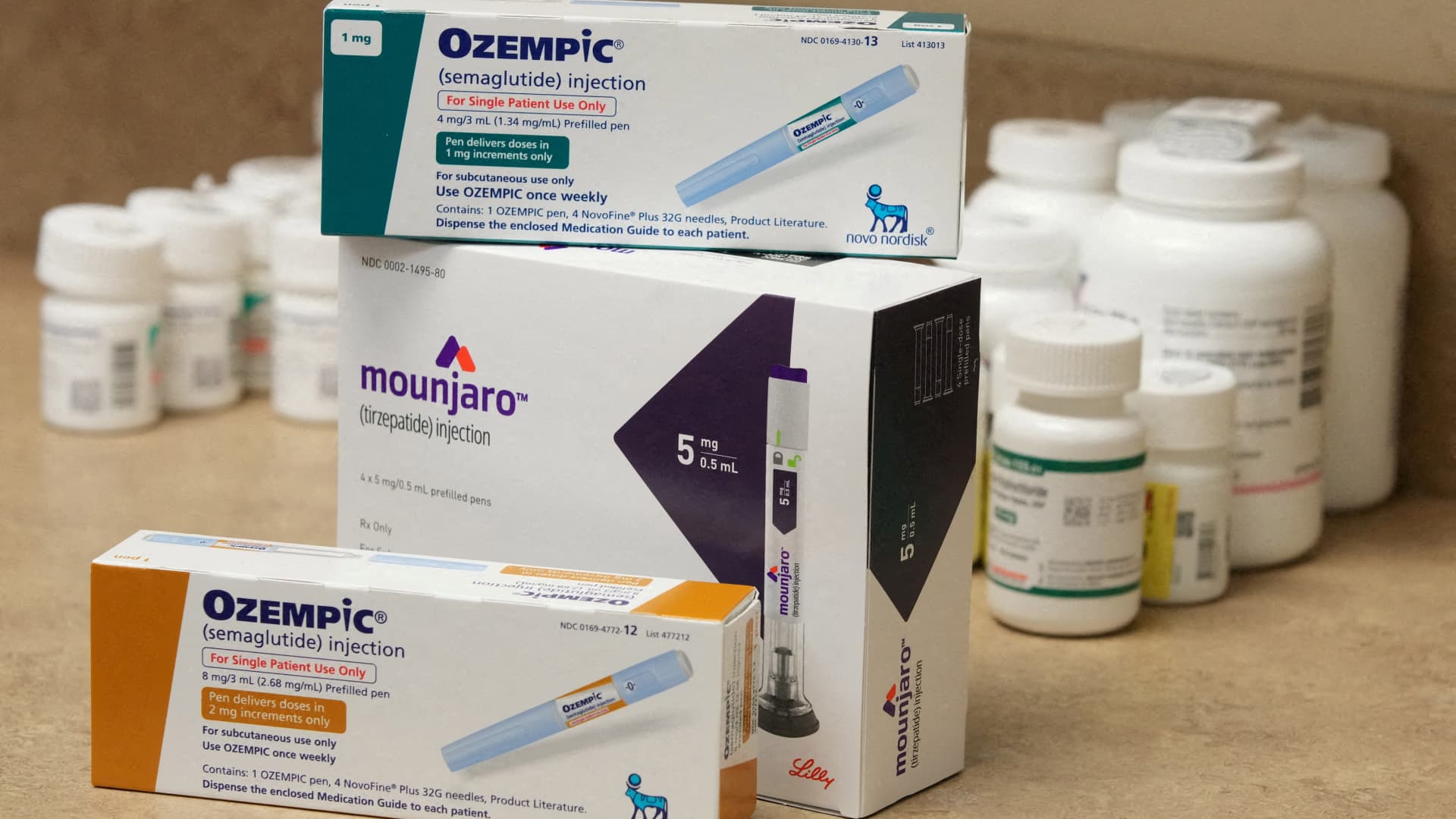
While Novo Nordisk stock is having its worst year in 2025, down 50% year-to-date, Eli Lilly has become an investor favorite as the Indiana-based company's Zepbound and Mounjaro have shown steeper weight loss than Novo's Ozempic and Wegovy. Lilly has also taken the lead in new recipes in the United States.
On Thursday, Lilly released early late-stage data on its next-generation weight-loss drug, retatrutide. It works differently than existing injections and appears to be more effective as it targets three different appetite-regulating hormones, as opposed to one or two like Wegovy and Zepbound.
Lilly's stock has held up better as investors see its portfolio as more likely to translate into financial returns, and the company also has a diverse portfolio that goes beyond diabetes and weight loss treatments.
Meanwhile, Zeeland shares, like Novo's, peaked in mid-2024, but gains have moderated as bets are also placed elsewhere. Just last month, it halted development of a dual GLP-1/GLP-2 agonist called dapiglutide, citing a crowded market for obesity drugs. Instead, Zealand indicated it would focus resources on candidates with greater potential for clinical differentiation.
Big Pharma names like AstraZeneca, amgen and Pfizer Everyone expects their own drug candidates to take a chunk of market share from Lilly and Novo, as do clinical-stage players like Structural therapeutics and Viking therapeutics.
The market gives credit to Lilly but underestimates innovation elsewhere, according to Morningstar's Karen Andersen. “While we see Lilly having more than 50% global share for the foreseeable future, we believe its share will stabilize as Novo and other competitors launch next-generation drugs,” he wrote in a November note. “The consensus fails to appreciate the potential of these drugs.”
Zealand Pharma shares down almost a third in 2025
Separately, Zealand announced an agreement with Chinese biotechnology company OTR Therapeutics to develop oral small molecule treatments for metabolic diseases. Under the agreement, OTR will receive $20 million upfront, with an additional $10 million if certain conditions are met, as well as up to $2.5 billion related to development, regulatory and commercial milestones.
UBS analysts called the partnership “an interesting move.”
“Ahead of a catalyst-rich 2026 with the survodutide P3 assay and petrelintide P2 readout, we will look at expectations for these readouts, the commercialization strategy and any color on the potential pricing of survodutide, and how Zealand sees potential differentiation for OTR small molecule drugs versus other oral GLP-1s already close to the market,” the analysts said.
Zeeland also said it will open a new research site in Boston that will combine its expertise in peptide drugs with AI-powered drug discovery.