File photo: The headquarters of the United States Drug and Food Administration (FDA) is seen in Silver Spring, Maryland, November 4, 2009.
Jason Reed | Reuters
A version of this article appeared for the first time in the Healthy Bulletin returns from CNBC, which brings the latest medical care news directly to its entrance tray. Subscribe here To receive future editions.
Food and medication administration took a new step to take energetic measures against the use of cheaper versions of popular weight loss and diabetes medications.
While it is good news for US consumers, it is not exactly a victory for the main GLP-1 manufacturers Eli Lilly and Novo Nordisk. The shares of both companies fell more than 2% on Friday after the announcement.
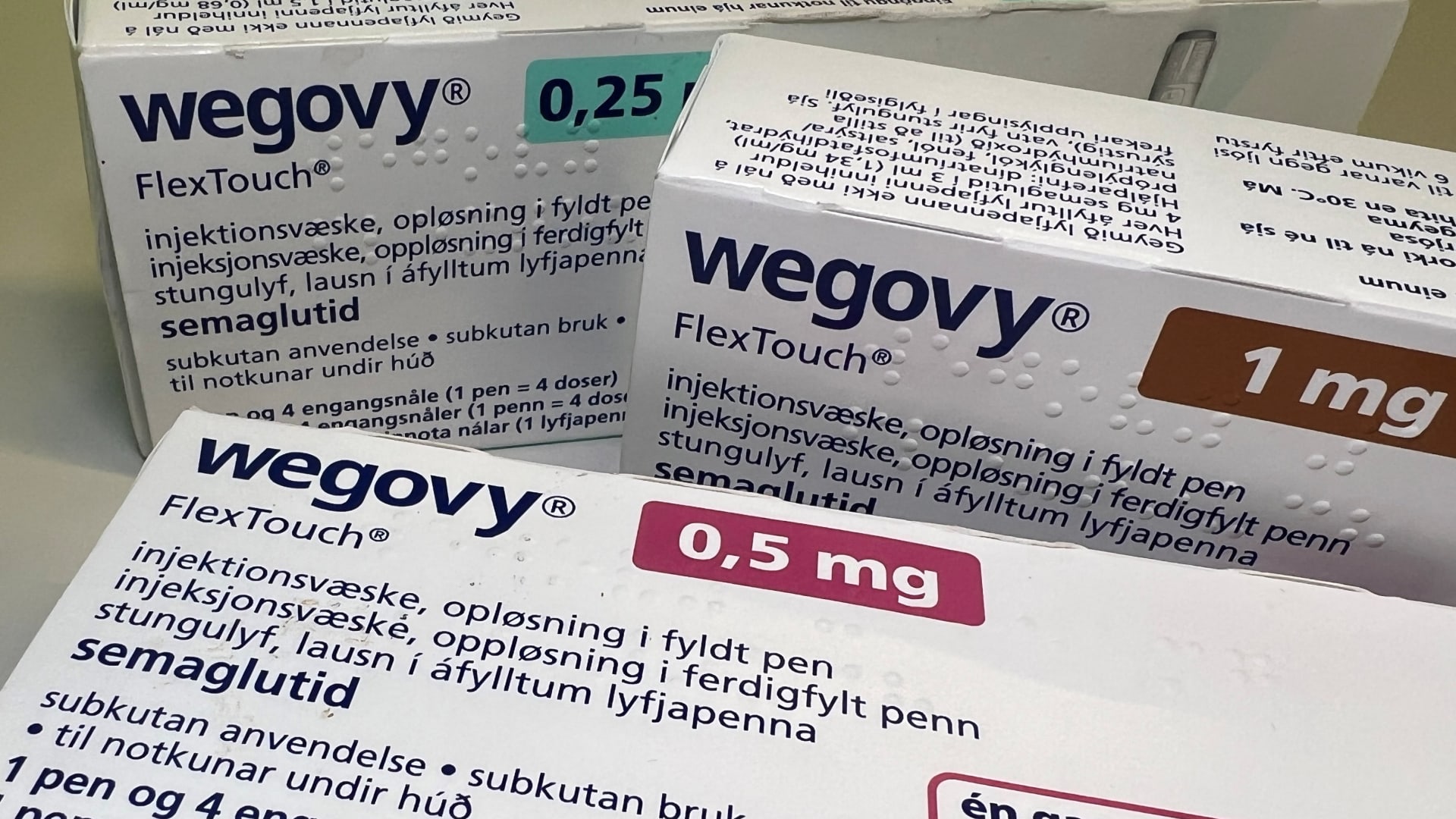
The FDA said on Friday that it will publish a “green list” of RAW GLP-1 ingredients of foreign suppliers whose facilities have been inspected by the agency and considered fulfilled the “rigorous” standards of the United States. Composite pharmacies use the so-called active pharmaceutical ingredients, or API, to make GLP-1 copies.
The list currently includes more than three dozen suppliers and their ingredients, but their names are written. Most of these suppliers seem to be in China, while others are based in Belgium, Italy, Canada and India, among other countries.
Meanwhile, the API of other suppliers will be “subject to detention without physical examination” if the US is imported to the US, according to an FDA statement. The FDA said that it aims to block the importation of potentially dangerous versions of the APIs used in LPG-1 compounds.
Consumers mass those compound treatments in recent years, since insurance coverage and the supply of brand obesity medications such as Wegovy and Eli Lilly's Zepbound's Zepbound were limited. Since then, companies have resolved that shortage. The FDA has previously identified serious concerns with versions composed of semaglutida and pullzepatida, the APIs used in Wogovy and Zepbound, respectively, as dosing errors that result in hospitalizations.
“By strengthening the supervision of imported APIs and taking energetic measures against illegal drugs entering the US.
But some analysts said that the new FDA effort is arrested aggressively aggressively the use of compounds.
Here is the BMO analyst Markets Evan Seigerman: The list “is probably positive for patient but negative” for Novo Nordisk and Eli Lilly actions.
The green list “recognizes the challenges of the composition without putting a full stop posture that will seek to end in general the products LGP-1,” he wrote in a note on Friday. Seigerman said the FDA seems comfortable by allowing some compound versions in the market provided they meet quality standards, regardless of the availability of brand LPG-1.
But he said it is a major problem for Novo Nordisk than for Eli Lilly: the company in July cut its sales orientation for Wagovy, partly due to the competition of versions composed of drugs in the United States.
Seigerman said it is clear that Novo Nordisk and Eli Lilly need to trust the litigation to stop the production of LPG-1 compounds. In the last two years, both companies have taken legal actions against dozens of compound pharmacies, medium spas and other suppliers to prevent them from doing and selling imitators.
He said that Novo Nordisk files a lawsuit against the Telesalud Hims & Hers company, “it could be a Gamechanger.” HIMS & HERS continues to offer compound semaglutid.
In a statement, an Eli Lilly spokesman said that the FDA movement “is an important first step, but it must be done more.”
The spokesman said that composite pharmacies have already imported large amounts of illegal pull. The spokesman urged the FDA and other regulators to “do more to stop the illegal composition before more people are injured.”
In a separate statement, a spokesman for Novo Nordisk said that “it is important that the FDA take energetic measures against imports of” illegal semaglutid; API and take measures to protect patients from security risks raised when taking imitation medications made with non -authentic and substantial API, including the API that has already imported the US. UU. “
Do not hesitate to send any advice, suggestion, stories ideas and data to Annika in [email protected].
The latest in medical care technology: Apple announces two new health features for Apple Watch during the annual hardware event
Customers expect outside for the new iPhone 16 and the 10 Apple Watch series on the fifth Apple Store Avenue in New York City on September 20, 2024.
Timothy A. Clary | AFP | Getty images
Apple He presented new iPhones, Apple Watches and Airpods during an event at its Cupertino headquarters on Tuesday, and a couple of new remarkable features of health and dream of the heart is coming to see users.
The company has been pushing more deeply to medical care in recent years, and announced Apple Watch Series, which includes “the most complete health functions so far,” Apple said in a statement.
Users can receive alerts about possible hypertension, or high blood pressure, affecting approximately 1.3 billion people worldwide, the company said. High blood pressure can lead to serious health problems such as heart attack or stroke, heart failure, kidney disease and other conditions, according to the Mayo Clinic.
Apple has developed an algorithm that analyzes how blood vessels respond to the rhythms of the heart using the optical heart sensor of the clock. It works in the background and review the data during periods of one month, and Apple will notify users if you identify possible hypertension patterns.
It is important to note that the characteristic is not an official diagnosis and does not measure blood pressure directly. If users receive a hypertension notification, they must talk to their doctor, Apple said.
“We hope to notify more than 1 million people with non -diagnosed hypertension only in the first year,” said Dr. Sumbul Desai, Apple's vice president of health, in a pre -recorded video during the event.
Desai said Apple hopes that the United States drug and food administration will clear the “soon” feature. It will be available with Watchos 26 at Apple Watch Series 9 and posterior and Apple Watch Ultra 2 and posterior.
Users will also get access to a new sleep score function, whose objective is to help people understand the quality of their dream. The tool provides users with a 100 score depending on their consistency before bedtime, how often they wake up, the duration of their dream and how long they spend at each stage of sleep.
The sleep score will be available with Watchos 26. Users can find it in the SLEEP application and track their results over time in the health application.
“Apple Watch Series 11 is an indispensable partner who supports health, physical state, safety and connectivity of users throughout the day and night,” Stan NG, Vice President of Apple Watch and marketing of health products, said in a statement.
The Apple Watch 11 series starts at $ 399, and users can reserve it now. The clock is officially available as of September 19.
Read the full publication of Apple's blog here.
Do not hesitate to send any advice, suggestion, stories ideas and data to Ashley at [email protected].