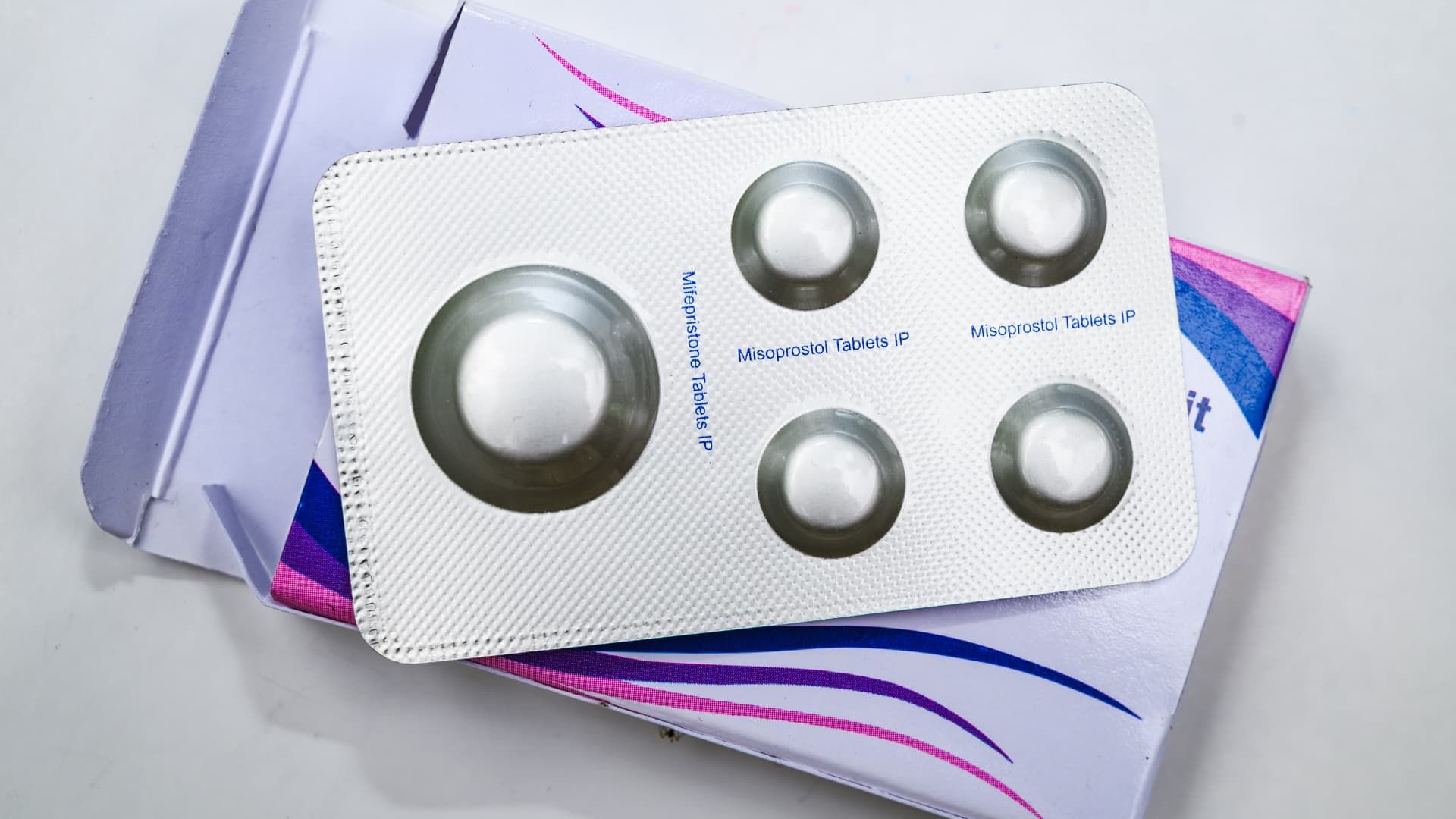
Mifepristone, also known as RU-486, is a medication typically used in combination with misoprostol to induce medical abortion during pregnancy and manage early miscarriage.
Soumyabrata Roy | Nurfoto | fake images
WASHINGTON – The Supreme Court on Thursday rejected a challenge to the abortion pill mifepristone, meaning the commonly used drug can remain widely available.
The court unanimously determined that the group of anti-abortion doctors who challenged the Food and Drug Administration's decisions facilitating access to the pill did not have legal standing to sue. Consequently, the complaint will be dismissed.
Justice Brett Kavanaugh, writing for the court, wrote that while the plaintiffs have “sincere legal, moral, ideological, and political objections to elective abortion and the FDA's relaxed regulation of mifepristone,” that does not mean they have a federal case.
The plaintiffs did not prove that they had suffered any harm, meaning that “federal courts are the wrong forum to address plaintiffs' concerns about the FDA's actions,” he added.
“Plaintiffs can present their concerns and objections to the President and the FDA in the regulatory process or to Congress and the President in the legislative process,” Kavanaugh wrote. “And they can also express their opinions about abortion and mifepristone to their fellow citizens, including in political and electoral processes.”
By dismissing the case on those grounds, the court avoided reaching a decision on the legal merits of whether the FDA acted legally in lifting several restrictions, including one that made the drug obtainable by mail, meaning the same issues still exist. They could return to court in another case.
Another regulatory decision that remains in place means women can still get the pill within 10 weeks of gestation instead of seven.
Likewise, the decision to allow health care providers other than doctors to dispense the pill will remain in place.
The ruling comes two years after the court, which has a 6-3 conservative majority, overturned the landmark abortion rights decision Roe v. Wade, which sparked a wave of new abortion restrictions in conservative states.
The court then suggested it would withdraw from the political debate over abortion, but as litigation over abortion access continues, justices continue to play a critical role.
The mifepristone dispute is not the only abortion case currently before the court. He must also decide whether Idaho's strict abortion ban prevents emergency room doctors from performing abortions when a pregnant woman faces dangerous complications.
Mifepristone is used as part of an FDA-approved two-drug regimen that is now the most common form of abortion in the United States.
Abortion is effectively banned in 14 states, according to the Guttmacher Institute, a research group that supports abortion rights.
The FDA had the backing of the pharmaceutical industry, which warned that any questions about the approval process from untrained federal judges could cause chaos and deter innovation.
The legal challenge was brought by doctors and other medical professionals represented by the conservative Christian legal group Alliance Defending Freedom.
Last year, Texas-based U.S. District Judge Matthew Kacsmaryk issued a sweeping ruling that completely invalidated the FDA's approval of the pill, sparking panic among abortion rights activists that would be banned throughout the country.
Last April, the Supreme Court stayed that ruling, meaning the pill remained widely available while the litigation continued.
In August, the New Orleans-based U.S. Court of Appeals for the Fifth Circuit limited Kacsmaryk's decision but upheld its conclusion that the FDA's decision to lift restrictions starting in 2016 was unlawful.
Both parties appealed to the Supreme Court. In December, the court accepted the Biden administration's appeal defending subsequent FDA decisions but opted not to hear the challenge to mifepristone's original 2000 approval.
The Supreme Court focused solely on subsequent FDA action, including the initial 2021 decision that made the drug available by mail, which ended last year.