Soup Images | Lightrocket | Getty Images
Pfizer On Thursday it announced it will move ahead with a once-daily version of its weight-loss pill, danuglipron, after seeing “encouraging” data in an ongoing early-stage trial.
The company evaluated several once-daily formulations of the drug and identified one with “the most favorable profile.” Pfizer said it plans to conduct studies in the second half of the year to identify the ideal dose of the drug.
Pfizer is one of several pharmaceutical companies vying for market share for a popular class of weight-loss and diabetes drugs, GLP-1 agonists. Some analysts expect the industry to be worth about $100 billion by the end of the decade.
The company's shares rose more than 3% in premarket trading on Thursday.
In December, Pfizer halted administration of a twice-daily version of danuglipron after patients had trouble tolerating the drug in a mid-stage study. At the time, the pharmaceutical giant said data from the phase one trial on the once-daily version would “inform us for the future.”
But investors have been pessimistic about the company’s potential in the GLP-1 space since it abandoned a different once-daily pill in June 2023. Those were among the setbacks Pfizer faced last year, on top of the rapid decline of its Covid business, which hammered its stock.
Pfizer has other experimental drugs in the early stages of development, including one for obesity. The company has not disclosed how those treatments will work.
Pfizer believes GLP-1s are just “the tip of the iceberg of what we're going to see in obesity,” Chief Executive Albert Bourla said during a conference in June.
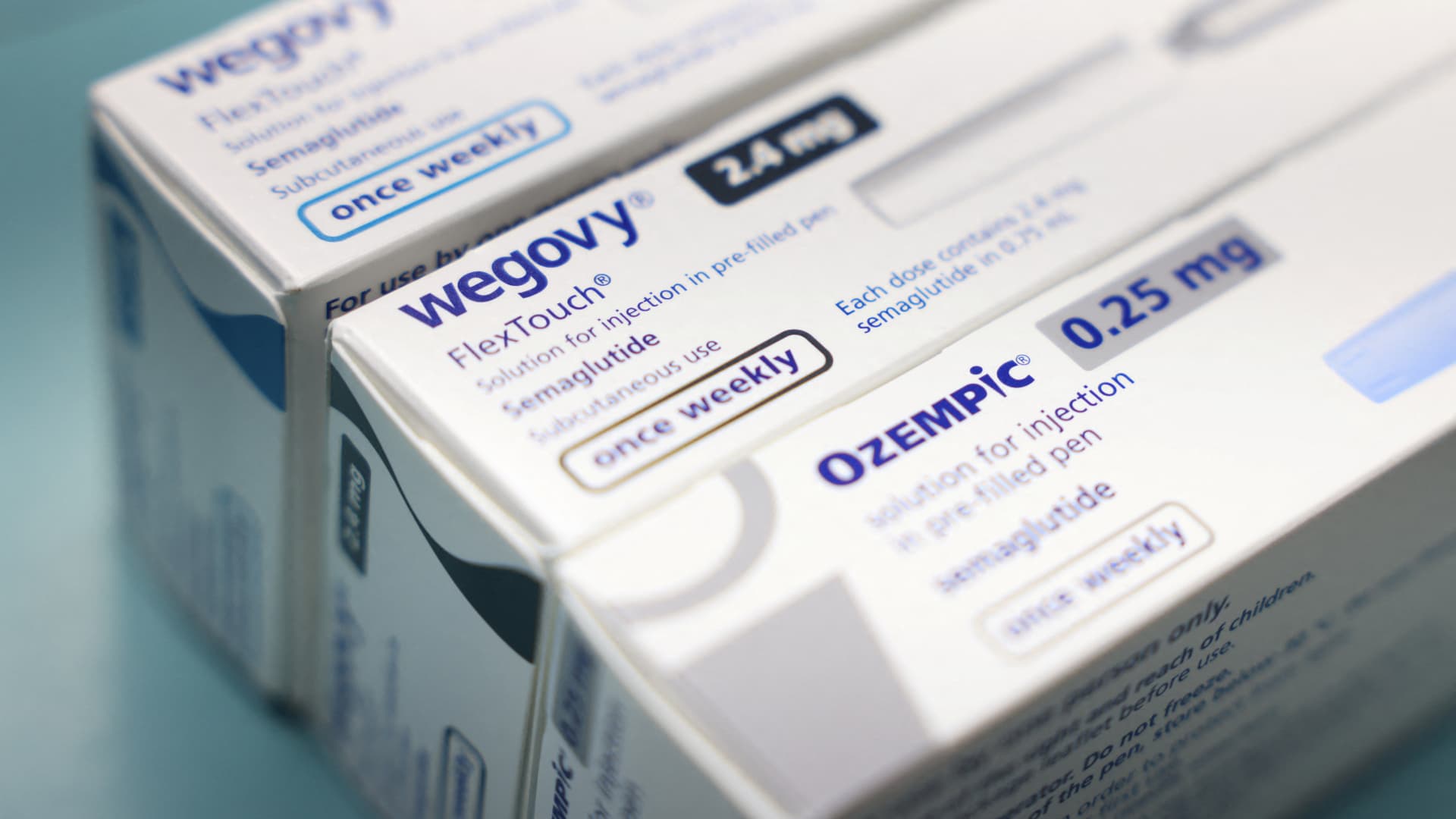
Pfizer's Danuglipron is a GLP-1 that promotes weight loss in the same way as 'New Nordisk'Wegovy injection and Ozempic diabetes treatment. The drugs mimic a single hormone produced in the gut called GLP-1, which sends signals to the brain when a person is full.
Novo Nordisk injections and Eli Lilly have increased enormously in demand over the past year despite their high prices and limited insurance coverage.
The pair, along with Pfizer and other drugmakers, have been racing to develop oral versions that are more convenient for patients to take and easier to manufacture, which could help ease supply shortages in the U.S.
Pfizer has not ruled out acquiring or partnering with a smaller obesity drug maker.
Bourla told reporters at a conference in January that the company was unlikely to buy an obesity treatment at a later stage of development, especially as the company focuses on cutting costs.
But he said Pfizer was looking at potential licensing deals for early-stage weight-loss drugs.
Pfizer's update on danuglipron comes days after the company said it is seeking a successor to its chief scientific officer, Mikael Dolsten, who is stepping down after more than 15 years at the drugmaker. Dolsten played a crucial role in the development of Pfizer's Covid vaccine.