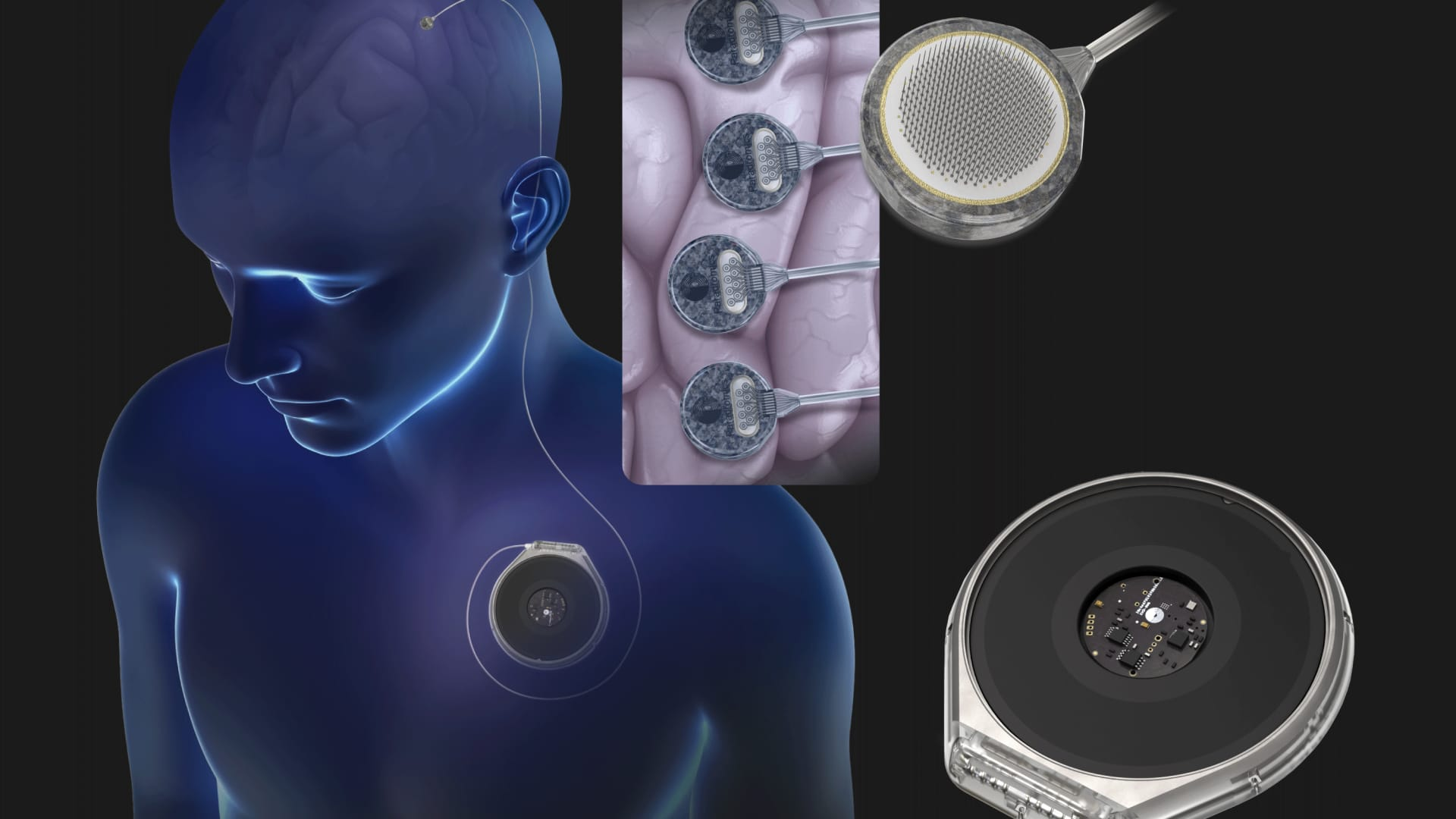
Neurotech startup Paradromics will test its brain implant next year, as the race to be a leader in the nascent brain-computer interface (BCI) space heats up.
“The brain is a super fascinating organ. We have about 85 billion neurons and each neuron is a million times slower than a computer chip. And yet the brain does incredible things,” said Matt Angle, director executive and founder of Paradromics, in an interview with CNBC Tech: The Edge.
“What that means is that if you want data to go in and out of the brain, you have to be able to talk to a bunch of different neurons simultaneously. And that's where the emphasis is on building these high-speed, high-capacity data transmission systems.” data. tariff devices come from,” he added.
The trial would follow competitor Neuralink, which implanted a chip in a patient's brain in March this year. The company, co-founded by Elon Musk, later revealed that part of its brain implant malfunctioned in the weeks after the procedure.
Founded in 2015, Paradromics has raised $87 million in venture investment and $18 million in crowdfunding to date. The Austin, Texas-based startup anticipates the devices will sell for about $100,000 each.
“The mission of paradromics is to transform otherwise untreatable brain health conditions into solvable technological problems. Basically, we are building a medical device to meet unmet needs,” Angle said.
While Angle anticipates the device will be able to treat a wide range of conditions, Paradromics will first focus on patients who have lost their ability to communicate, whether due to paralysis, amyotrophic lateral sclerosis or spinal injury.
“The reason we have chosen to focus on motor and speech is because they are very well-trodden in our research community and the science exists,” said Vikash Gilja, chief scientific officer at Paradromics.
“Paradromics can take science and apply appropriate engineering to take us from research to medical device,” he added.
Gilja told CNBC that the device will work wirelessly and will not require charging.
“The only thing you would have to do as a user is go through a short calibration routine to learn how to map from the electrical signals to the intent. But once that mapping is learned, the system can be used,” Gilja said.
Angle is hopeful that Paradromics will gain commercial approval to sell the product as soon as 2029, but not before.
“We see that the first million people who will get brain-computer interfaces will receive them to treat serious medical conditions,” Angle said.
“I think there might be a different conversation 20 years from now, and some of those devices might have consumer applications as well. But in the meantime, we're really focused on building safe, reliable, robust devices for people with physical and mental conditions.” .