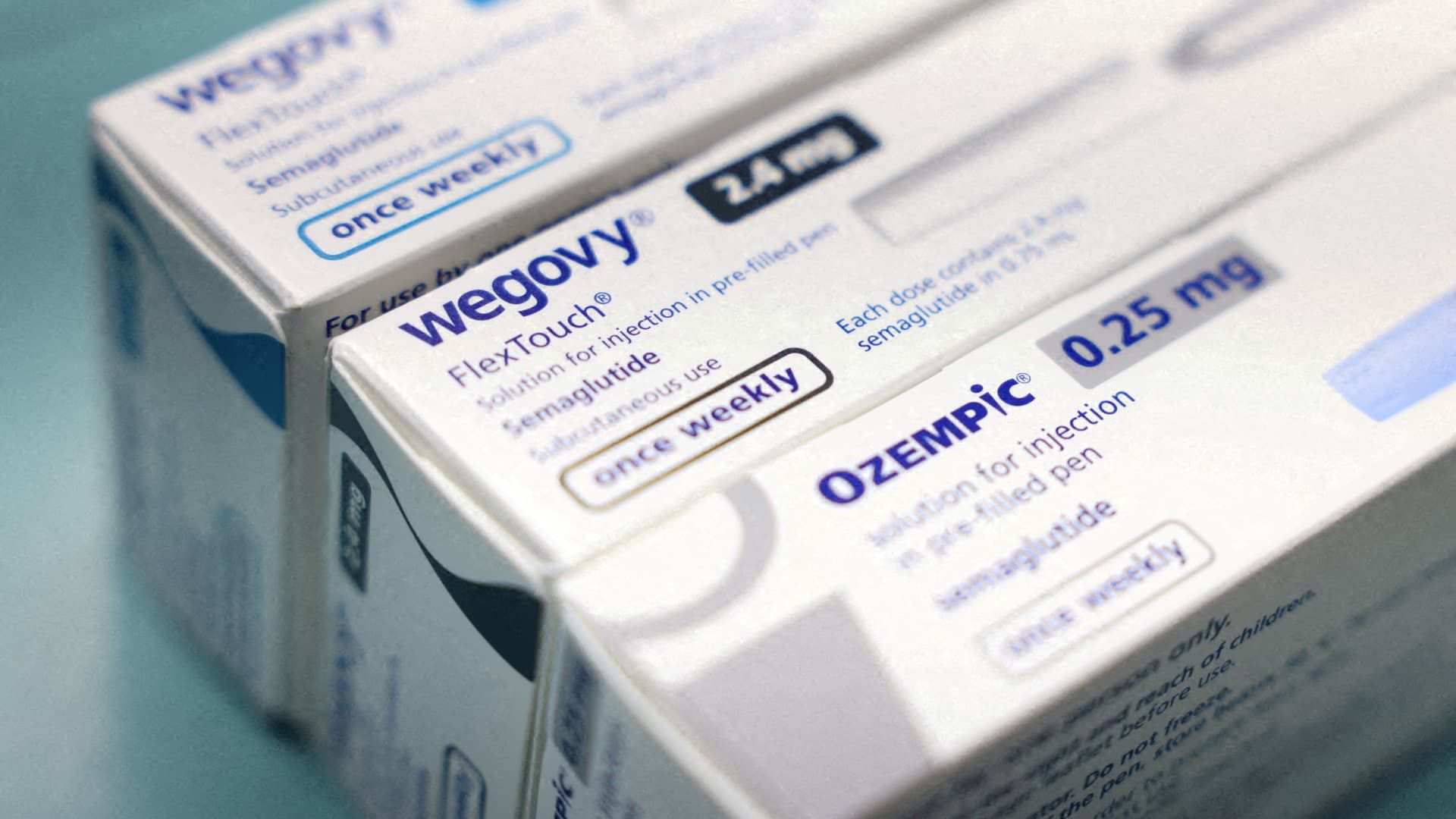
Nordisk On Tuesday it asked the Food and Drug Administration to stop compounding pharmacies from making unapproved and often cheaper versions of its popular weight-loss shot Wegovy and diabetes treatment Ozempic, arguing that the drugs are too complex to that those manufacturers produce them safely.
The FDA has yet to make a final decision on whether to ban compounded versions of semaglutide, the active ingredient in Ozempic and Wegovy. In a statement, the agency said it is reviewing the request and will respond directly to Novo Nordisk.
The move is Novo Nordisk's latest attempt to crack down on potentially harmful copies of semaglutide after it filed 50 lawsuits against various clinics, compounding pharmacies and other manufacturers over the past year. It comes as the Danish drugmaker tries to increase supplies of semaglutide to meet unprecedented demand in the US.
Patients have turned to compounded versions of semaglutide amid an intermittent shortage of brand-name drugs in the United States, which carry high prices of $1,000 a month before insurance and other reimbursements. Many health plans do not cover semaglutide for weight loss, making compounded versions a more affordable alternative.
Compounded medications are personalized alternatives to brand-name medications designed to meet the needs of a specific patient. When a brand-name drug is in short supply, compounding pharmacies can prepare copies of the drug if they meet FDA requirements.
The active ingredient in Wegovy and Ozempic, semaglutide, has been in intermittent shortage for the past two years. The lowest dose of Wegovy is currently in short supply, but all other doses of the drug and Ozempic are listed as available, according to the FDA's drug shortage database.
But Novo Nordisk on Tuesday night nominated semaglutide to the FDA's “Demonstrable Compounding Difficulties” lists, which include complex drugs that compounding manufacturers can't make, even during shortages, because they could pose security risks.
“Semaglutide products fit this description due to their inherent complexity and the potential dangers associated with attempting to combine them,” Novo Nordisk said in a statement.
The Danish drugmaker cited several risks with compounded versions of semaglutide, including unknown impurities, incorrect dosages, and cases where a compounded product did not contain semaglutide at all.
“These drugs are inherently complex to combine safely, and the risks they pose to patient safety far outweigh any benefits,” Novo Nordisk said in a statement. The company said its “goal with this nomination is to ensure that patients receive only safe and effective FDA-approved semaglutide products.”
The FDA previously warned about the risks of using compounded versions of so-called GLP-1, such as semaglutide. This refers to a class of medications that mimic hormones produced in the intestine to reduce a person's appetite and regulate their blood sugar level.
Earlier this month, the FDA said that compounded versions of semaglutide and similar drugs can be risky to patients because they are unapproved, meaning the agency does not review their safety, effectiveness and quality before they go on the market.
In August, the FDA also said it had received reports of patients who overdosed on compounded semaglutide due to errors such as self-administering incorrect amounts of a treatment.
Both Wegovy and Ozempic are protected by patents in the United States and abroad, and Novo Nordisk and its rival Eli Lilly They do not supply the active ingredients of their medicines to outside groups. The companies say this raises questions about what some manufacturers sell and market to consumers.
Tirzepatide is the active ingredient in Eli Lilly's Zepbound weight loss injection and Mounjaro diabetes treatment.
Like Novo Nordisk, Eli Lilly has sued several weight loss clinics, medical spas and compounding pharmacies across the United States over the past year.
Notably, the FDA removed tirzepatide from its shortage list in early October, after more than a year, even as some pharmacies say they are still struggling to stock brand-name versions of that drug. A trade group representing some compounding manufacturers sued the FDA, prompting the agency to say it will reconsider its decision to remove tirzepatide from its shortage list.