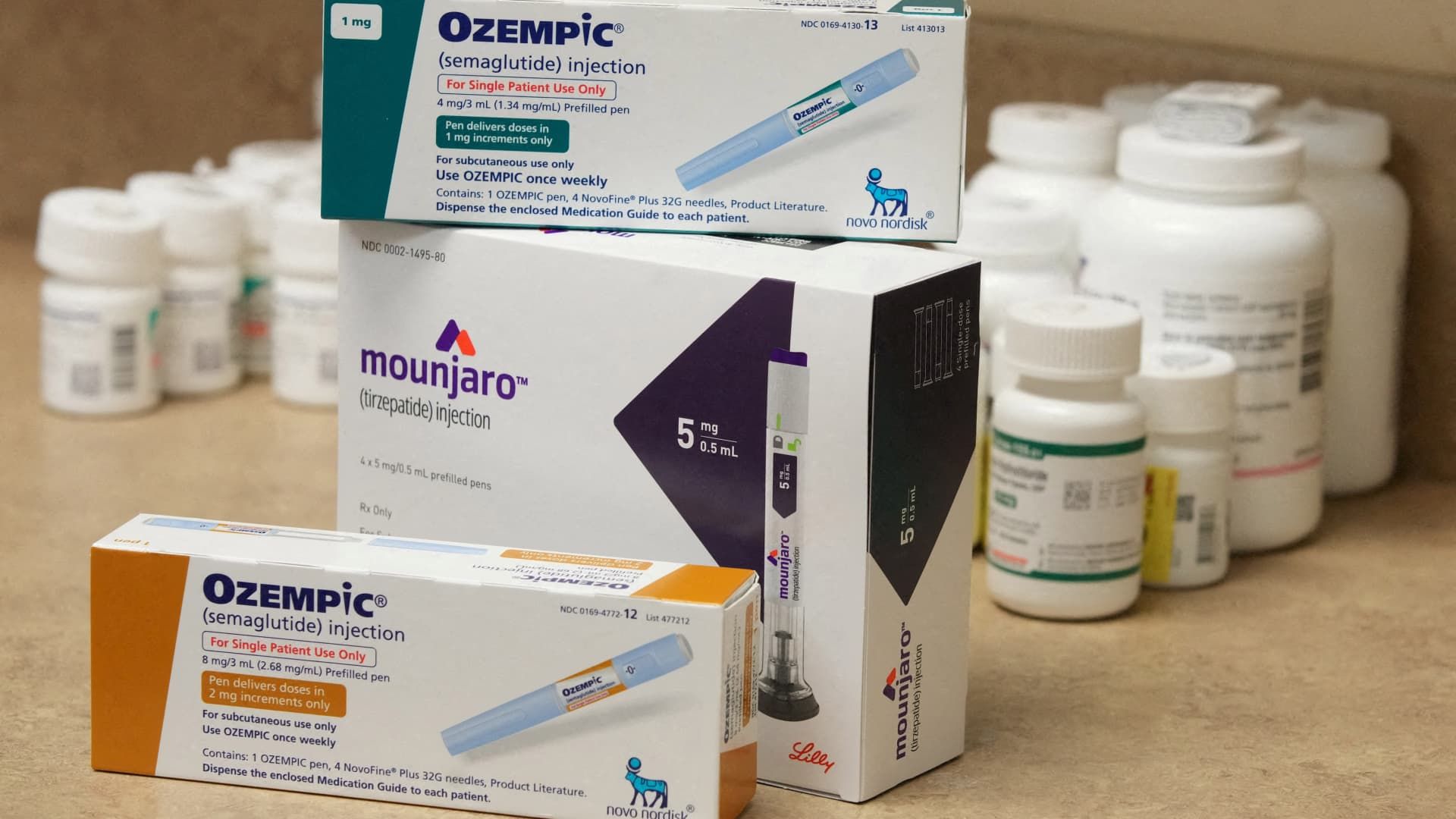
Good afternoon! All eyes are once again on Novo Nordisk and Eli Lilly as Wall Street looks for signs they can address one of the biggest hurdles they faced last year. Neither company has enough supply to meet the insatiable demand for their diabetes and weight loss medications.
One month into 2024, the two drugmakers still haven't fully resolved those supply issues. They don't expect to do it anytime soon. Still, Eli Lilly and Novo Nordisk appear to be making some encouraging progress.
Eli Lilly achieved its goal of doubling its capacity to produce injectable incretin drugs by the end of 2023, the company's chief financial officer, Anat Ashkenazi, said during an earnings call Tuesday. Anti-incretin medications, such as Eli Lilly's Zepbound weight-loss treatment and Mounjaro diabetes injection, mimic hormones produced in the gut that suppress a person's appetite and regulate blood sugar.
Ashkenazi added that Eli Lilly will try to increase capacity with “equal urgency” this year. The company expects the most significant production increases to occur in the second half of 2024.
By that time of year, Eli Lilly expects its production of salable doses of anti-incretin drugs to be at least 1.5 times greater than its production of those doses in the second half of last year, Ashkenazi added.
Among the company's efforts to expand production is its new manufacturing facility in North Carolina. Ashkenazi said the plant will begin producing anti-incretin drugs in late 2024, with products available to ship in 2025.
The company still expects demand for anti-incretin drugs to outstrip supply this year as it works to ramp up production, Ashkenazi said.
Eli Lilly wasn't the only weight-loss drug maker to see positive developments in supply over the past week. Novo Nordisk did it too.
Novo Holdings, which owns nearly 77% of Novo Nordisk's voting shares, said Monday it will acquire drugmaker Catalent in a $16.5 billion deal.
Catalent is critical to Novo Nordisk because it is the primary supplier of fill and finish work, which involves the filling and packaging of syringes and injection pens, for Wegovy.
Novo Nordisk will then buy three of Catalent's manufacturing plants from Novo Holdings for $11 billion. Novo Nordisk said the purchase will gradually increase its filling capacity starting in 2026.
“Overall, we believe this will further unlock supply, which is the key bottleneck for this market,” Yuri Khodjamirian, chief investment officer at Tema ETF, told CNBC in reaction to the Catalent deals on Monday. In November, Tema launched an ETF whose key holdings include companies benefiting from the hype around weight-loss drugs.
The deal also means Novo Nordisk can better control the quality of Wegovy's supply, which has previously been an issue at Catalent's facilities, Khodjamirian added.
For example, Catalent's Brussels factory that fills Wegovy injection pens suffered several failures in recent years and had to close twice, Reuters reported in July, citing FDA inspection documents.
The deal comes as Novo Nordisk attempts to take broader steps to improve supply this year.
Last week, the Danish drugmaker also said it had more than doubled its supply of lower-dose versions of its Wegovy weight-loss shot in January compared with previous months. Supply shortages forced Novo Nordisk to restrict availability of those lower doses in the U.S. since May.
But why are those lower doses important? This is because people are supposed to start Wegovy at a low dose and gradually increase the size over time to mitigate side effects like nausea. Therefore, more of those “initial” low doses means more new patients can begin treatment with Wegovy.
The company plans to “gradually” increase overall Wegovy supply over the remainder of the year, executives added on the company's fourth-quarter earnings conference call Wednesday.
The latest in health technology
The brain as the next frontier
The field of implantable neurotechnology is heating up.
Last week, Elon Musk announced that his startup Neuralink implanted its brain-computer interface in a human patient for the first time. musk said The recipient is “recovering well,” according to a post on its X social media site, but the famously secretive neurotechnology company did not share any other details publicly.
Neuralink is developing a brain implant, called a brain-computer interface, or BCI, designed to help paralyzed patients control external technologies using only their minds. It sounds like something out of a science fiction movie, but several companies such as Synchron, Precision Neuroscience, Paradromics and Blackrock Neurotech have developed systems with these capabilities.
“Imagine if Stephen Hawking could communicate faster than a typist or an auctioneer,” Musk wrote. “That's the goal.”
Musk's announcement marks a major milestone for Neuralink, but it's not unexpected. Neuralink began recruiting patients for its first-in-human clinical trial in the fall after receiving approval from the U.S. Food and Drug Administration to conduct the study in May, according to a post on the company's blog.
The road to market is long for medical device companies. Neuralink will need to conduct further testing demonstrating the safety and effectiveness of its BCI before it can get the final seal of approval from regulators.
Many competing BCI companies, such as Synchron, are also working to bring products to market. On Thursday, Synchron announced it has acquired a minority stake in German manufacturer Acquandas, which will help the company ramp up production of its flagship BCI to prepare for commercial demand.
Synchron's stent-like BCI is delivered to the brain through the patient's blood vessels. So far, the company has implanted six patients in the US and four patients in Australia.
“We believe there are millions of people with paralysis who need this technology and we are preparing to produce in high volumes,” Synchron CEO Tom Oxley told CNBC in an interview.
Feel free to send tips, suggestions, story ideas, and facts to Annika at [email protected] and Ashley at [email protected].