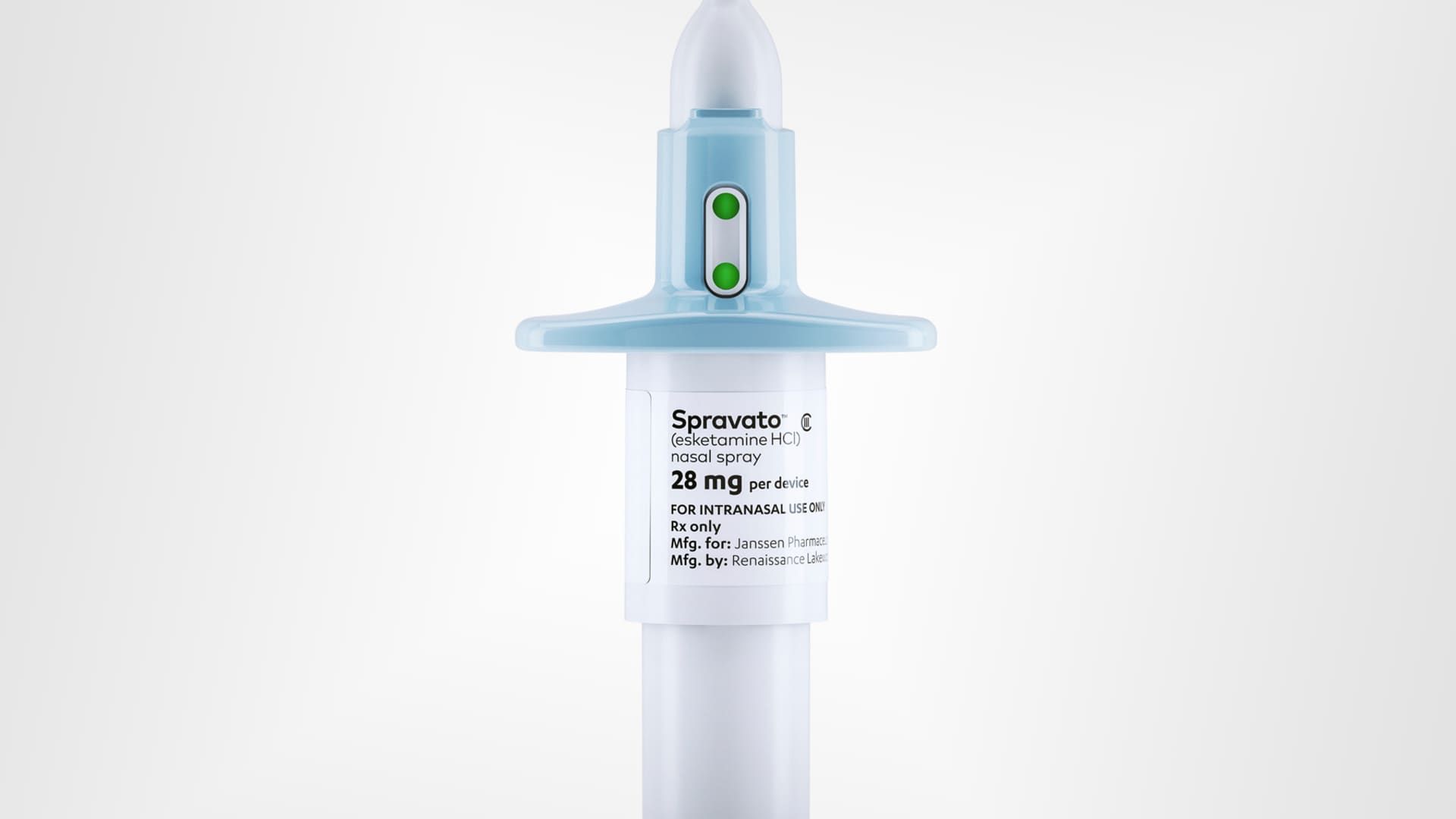
This photo provided by Janssen Global Services shows Spravato nasal spray.
Janssen Global Services via AP
The Food and Drug Administration on Tuesday approved Johnson & Johnson's nasal spray will be used only in adults with major depressive disorder that is difficult to treat, as sales of the drug increase.
The spray, called Spravato, is now the first stand-alone therapy for treatment-resistant depression – that is, when trying at least two standard treatments does little or nothing to improve a patient's depression symptoms.
Previously, Spravato was approved for use in the US along with an oral antidepressant both for treatment-resistant depression and for people with major depressive disorder who experience thoughts of suicide or harm. The drug first entered the US market in 2019.
“We want to recognize that this is a drug that treats a disease that [when] “If left untreated, depression is potentially fatal,” Bill Martin, head of J&J's global neuroscience therapeutics area, said in an interview.
About a third of the estimated 21 million American adults with major depression struggle with symptoms (such as persistent feelings of sadness, sleep disturbances, lack of energy and thoughts of death or suicide) that do not respond to treatment, according to some estimates.
“For the first time, we now have an option that gives freedom to patients,” said Dr. Gregory Mattingly, a physician and president of the Midwest Research Group who participated in Spravato's original clinical trials.
Its center in St. Louis, Missouri, has treated more than 6,000 patients with the drug and just over 100 people currently take it there. This is one of 3,000 outpatient treatment centers in the U.S. that are certified to administer Spravato, by J&J's count.
Mattingly said patients can now choose to take Spravato with or without an oral antidepressant, especially if those pills do not improve their symptoms and cause undesirable side effects, such as weight gain and sexual problems.
J&J's Martin said the approval provides “an avenue for caregivers and their patients to truly optimize and personalize the treatment paradigm for each individual” and determine the best way to manage the disease.
According to Martin, this could potentially “increase the number of patients who could benefit” from Spravato.
Spravato is on track to become a blockbuster product, with the drug generating $780 million in sales through the first nine months of 2024 as doctors become more comfortable using it, according to J&J's third-quarter earnings. The company has even higher expectations for its growth, telling investors in December that it expects sales to rise to between $1 billion and $5 billion a year.
This is a boon for J&J as it prepares for an upcoming patent expiration and new prices negotiated with Medicare to pressure sales of its best-selling inflammatory treatment, Stelara.
The approval is based on a phase four trial, which showed that Spravato only improved depressive symptoms that began about 24 hours after treatment and lasted at least a month. The company has said the safety profile was consistent with previous clinical data on the use of Spravato in combination with oral antidepressants.
Martin said this demonstrates “not only rapid symptom relief, but also long-lasting symptom relief” when patients take Spravato alone.
Spravato's long road to rapid growth
Spravato broke new ground in 2019 as the first new treatment for major depression to win FDA approval in more than three decades. The drug is related to ketamine, a common anesthetic that can have hallucinogenic effects and is sometimes misused for recreational purposes. J&J made it into a nasal spray so it reaches the brain quickly.
Spravato “activates neural networks in a different way,” Mattingly said.
“Our standard oral antidepressants took weeks or months to see if they were going to work,” he added. “Very often, the same day, the next day, the next day, people can already start to feel somewhat better” with Spravato.
Spravato's warning label warns of the risk of sedation and dissociation, respiratory depression, suicidal thoughts, and abuse or misuse of the medication, among other possible side effects. Because of this, Spravato is only available through a restricted program, meaning it cannot be purchased in a pharmacy and is only administered in certified healthcare settings under strict supervision.
Users of the medication should also be supervised by a healthcare professional for two hours following administration.
Spravato's launch got off to a slow start, especially as pandemic-related challenges complicated arrangements for necessary medical oversight of the drug. But J&J began marketing more Spravato after in-person doctor visits became the norm again and doctors became more aware of its benefits.
“The mental health community wasn't really used to doing procedures at that time. We weren't used to having a reserved space. We weren't used to thinking about how to do Spravato,” Mattingly said. “I think the good news is that now we've all seen the benefits for our patients. Many of us have become strong advocates” for it.
Five years of real-world data on the drug and a head-to-head study demonstrating Spravato's superior efficacy to an oral antidepressant also gave doctors greater confidence in the treatment, according to J&J's Martin.