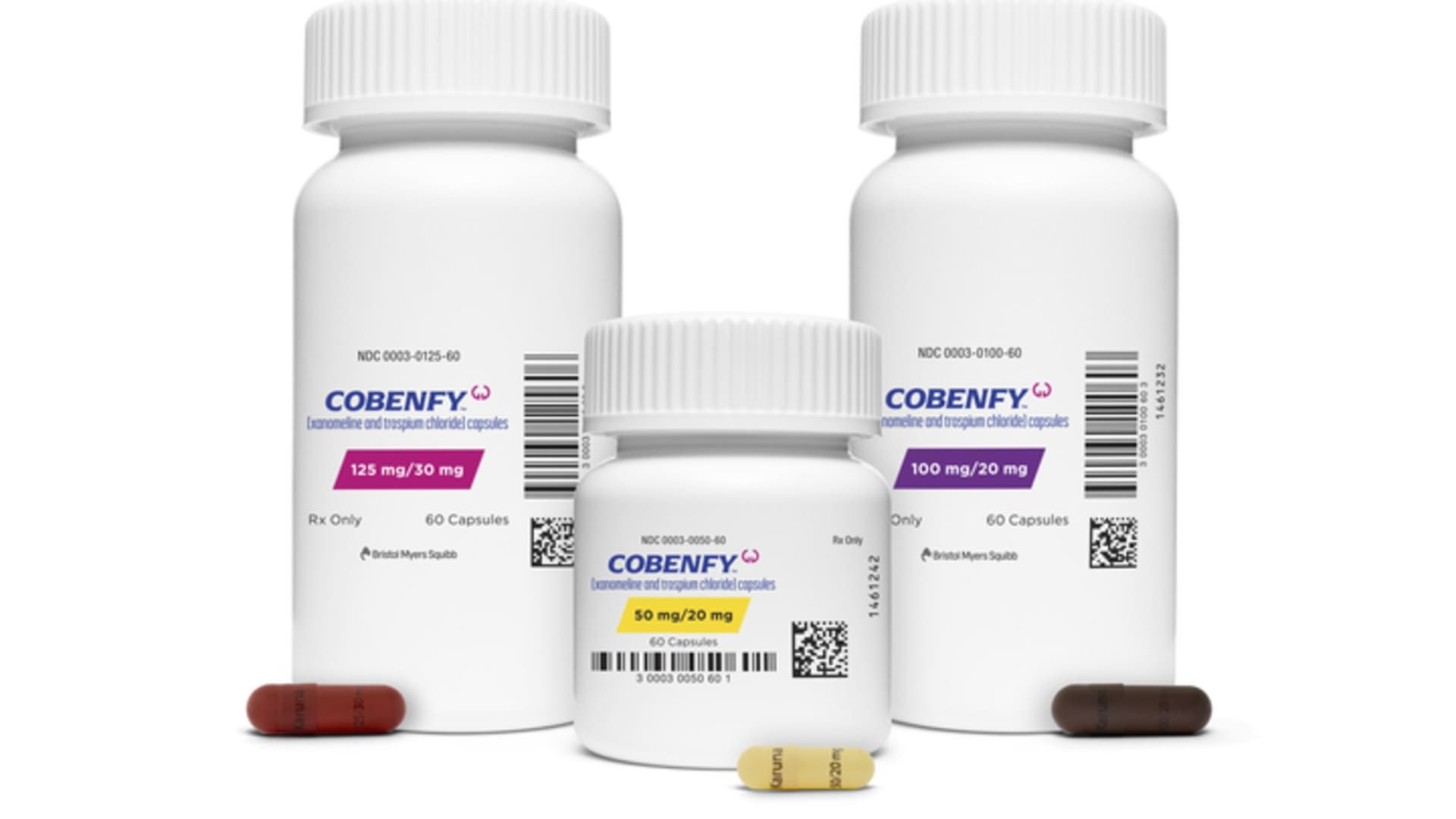
Bristol Myers Squibb's Cobenfy drug
Courtesy: Bristol Myers Squibb
The Food and Drug Administration on Thursday approved Bristol-Myers SquibbCobenfy, the long-awaited schizophrenia drug, the first novel type of treatment for this chronic and debilitating mental disorder in more than seven decades.
Schizophrenia affects the way a person thinks, feels and behaves and can cause paranoia, delusions, hallucinations and changes in emotions, movements and behaviour. These symptoms can disrupt a patient's daily life, making it difficult to go to school or work, socialize, and complete other daily activities. Most people are diagnosed between their late teens and early 30s.
Bristol Myers Squibb expects the twice-daily pill, which will be sold under the Cobenfy brand, to be available by the end of October, executives told CNBC. The drug is a much-needed new option for the nearly 3 million adults in the U.S. living with schizophrenia, some medical experts say.
Only 1.6 million of these patients receive treatment for this condition and 75% of them stop taking existing medications in the first 18 months because they have difficulty finding treatments that are effective or easy to tolerate, according to the drug manufacturer.
Cobenfy could also be a big long-term sales opportunity for Bristol Myers Squibb, which faces pressure to offset potential lost revenue from best-selling treatments whose patents are set to expire. The drug comes from the huge $14 billion acquisition of biotech company Karuna Therapeutics late last year.
In a July research note, Guggenheim analysts said they view Cobenfy as a “long-term, multibillion-dollar opportunity” for the company. But they said the drug will likely have a slow launch, so it may not contribute significantly to Bristol Myers Squibb's revenue in 2024 and 2025.
“I think there is a potentially transformative moment in the way we treat and talk about schizophrenia. And what we have, unfortunately, is an often underserved population that is not getting the attention it deserves from a research and healthcare perspective,” Andrew Miller, founder and former president of research and development at Karuna Therapeutics and now an advisor at Bristol Myers Squibb, told CNBC.
“I think the biggest moment will be five or 10 years from now, when we look back and say we've really made a difference,” he continued. “We've helped people, we've improved outcomes, we've given caregivers and doctors another tool they can use.”
Cobenfy will cost $1,850 for a monthly supply or $22,500 annually before insurance and other reimbursements, Bristol Myers Squibb executives said.
They said the price is in line with existing brand-name treatments for oral schizophrenia and that they expect most patients, particularly those enrolled in Medicare and Medicaid plans, to have minimal out-of-pocket costs for the drug. According to Bristol Myers Squibb, about 80% of patients suffering from this condition are covered by government insurance.
The company intends to launch a program aimed at helping patients pay for Cobenfy, executives added.
It's not yet clear to what extent that program will increase access for the uninsured.
Cobenfy will have to compete with some existing schizophrenia drugs – called antipsychotic treatments – with lower list prices, particularly generic imitations of brand-name treatments. For example, uninsured patients can get the generic version of an antipsychotic treatment called Abilify for as little as $16 for 30 tablets once a day with free GoodRx coupons.
Existing schizophrenia medications work by directly blocking dopamine receptors in the brain to generally improve patients' symptoms.
But they come with a long list of potential serious side effects that can cause patients to stop treatment, including weight gain, excessive fatigue, and involuntary, uncontrollable movements. According to WebMD, approximately one-third of people with schizophrenia are also resistant to conventional antipsychotic treatments.
Cobenfy is the first approved treatment in a new class of drugs that do not directly block dopamine to improve schizophrenia symptoms, Dr. Samit Hirawat, chief medical officer at Bristol Myers Squibb, told CNBC.
He said one part of Cobenfy is a drug called xanomeline, which activates certain muscarinic receptors in the brain to decrease dopamine activity without causing the side effects associated with antipsychotics. The second part of Cobenfy is called trospium, which reduces the gastrointestinal side effects related to xanomeline, such as nausea, vomiting, diarrhea, and constipation.
“Most of these patients have already been through one or two of these products,” Adam Lenkowsky, chief marketing officer at Bristol Myers Squibb, told CNBC. “So the excitement we're hearing from doctors is the opportunity for a patient to receive treatment without seeing side effects but also getting unprecedented efficacy.”
Lenkowsky said the company hopes Cobenfy will eventually become the standard treatment for schizophrenia as doctors learn more about the drug and become more comfortable prescribing it to patients.
But the price could limit the drug's use to patients who have already tried and failed other existing treatments, said Nina Vadiei, associate clinical professor of pharmacotherapy and translational sciences at the University of Texas at Austin School of Pharmacy.
“If it were up to me, I wouldn't necessarily say we have to try said Vadiei, a clinical psychiatric pharmacist who cares for patients with schizophrenia at San Antonio State Hospital.”
Test results and upcoming research.
The approval was based on data from three clinical trials that compared Cobenfy to a placebo, as well as two longer-term studies that examined how safe and tolerable the drug is for up to a year. Cobenfy met the primary goal of all three trials: significantly reducing schizophrenia symptoms compared to a placebo, according to Bristol Myers Squibb.
In the studies, Cobenfy caused mostly mild to moderate side effects, which were primarily gastrointestinal and dissipated over time, Miller said.
Bristol Myers Squibb said Thursday's approval for schizophrenia may be just the beginning for Cobenfy.
For example, the company has late-stage clinical trials underway examining Cobenfy's potential in treating patients with psychosis suffering from Alzheimer's disease. Bristol Myers Squibb said it hopes to publish data from those studies in 2026.
The company also plans to study Cobenfy's potential to treat bipolar mania and irritability associated with autism.
“When we think about Cobenfy, we think of it as multiple indications packaged into one product… because we're actually developing the drug not just for schizophrenia but for six other indications,” Hirawat said, referring to other potential uses for the drug.
— CNBC's Angelica Peebles contributed to this report.