The Dexcom logo is seen on a smartphone screen and in the background.
Pavlo Gonchar | SOPA Images | Light rocket | fake images
dexcom announced Tuesday that its new over-the-counter continuous glucose monitor called Stelo has been cleared for use by the U.S. Food and Drug Administration and will be available for purchase online this summer.
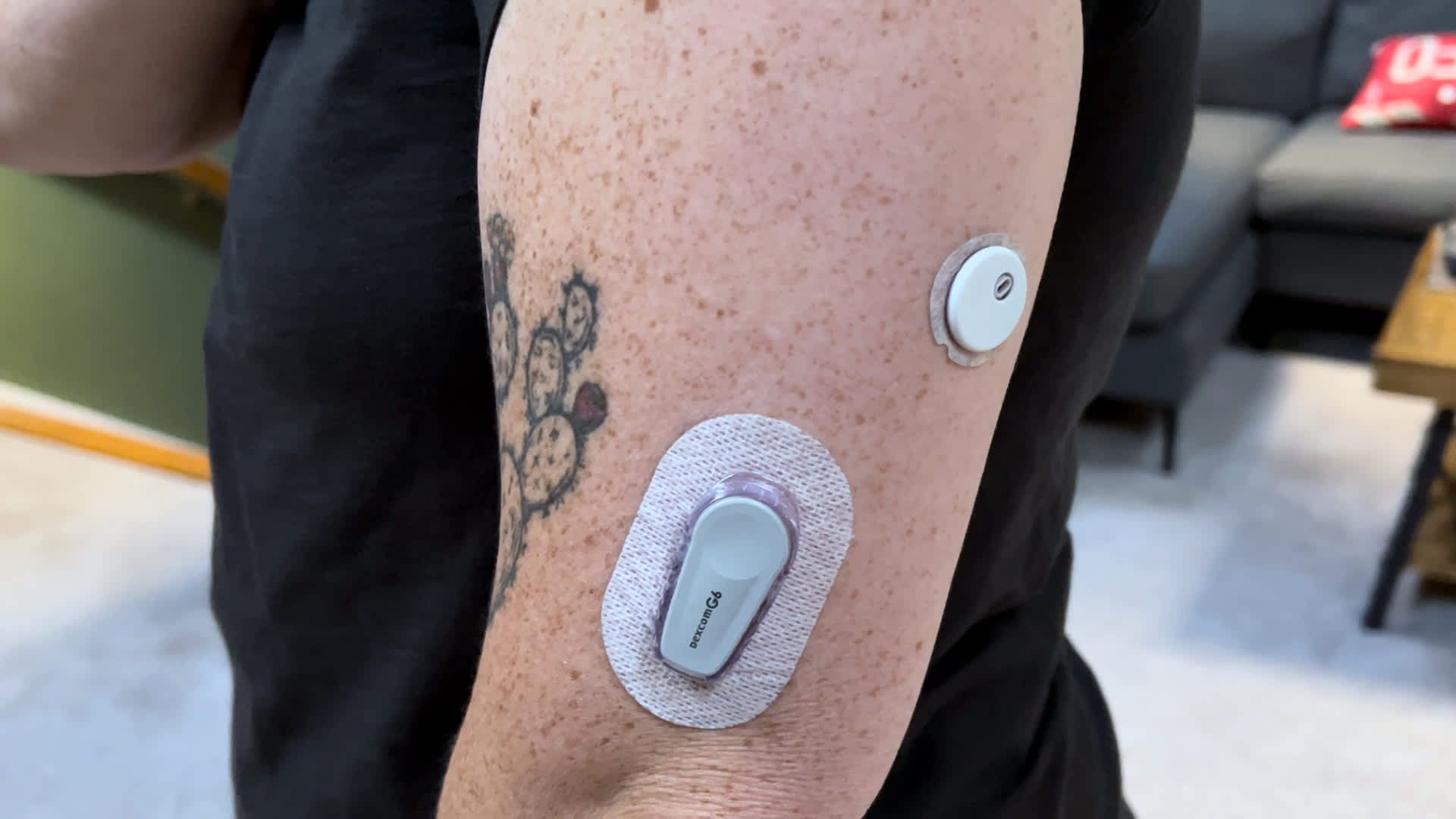
Continuous glucose monitors, or CGMs, are small sensors that pass through the skin to track glucose levels in real time. They are primarily used by patients with diabetes, as information is sent wirelessly to a smartphone, which can help alert users, their families, and their doctors to emergencies.
Dexcom's new CGM is designed for patients with type 2 diabetes who do not use insulin, and is the first glucose biosensor that does not require a prescription, according to a statement Tuesday. This means Stelo will be accessible to people who do not have MCG insurance coverage, Dexcom said.
There are more than 25 million patients with type 2 diabetes in the U.S. who do not use insulin, according to the Dexcom statement. While Dexcom's current G7 CGM system is available for this population, patients must obtain a prescription for it. As a result, it is not easily accessible to all patients with type 2 diabetes.
“CGMs can be a powerful tool to help control blood glucose. Today's authorization expands access to these devices by allowing people to purchase a CGM without the involvement of a healthcare provider,” said Dr. Jeff Shuren, director of the Center for Devices and Radiological Health, said in a statement.
Dexcom shares rose more than 2% in extended trading on Tuesday.
Dexcom shared Stelo's name, as well as the fact that the device had been submitted to the FDA for review in February. The sensor will be worn on the upper arm and lasts up to 15 days before needing to be replaced, according to Dexcom's website.
Jake Leach, Dexcom's chief operating officer, told CNBC in February that Stelo will have its own unique platform and brand. The platform will be tailored to the needs of these type 2 patients, he said, meaning it will not include many of the alerts and notifications intended for diabetes patients who are at risk of experiencing more serious emergencies.
“It's designed to be a simpler experience,” Leach said in an interview. “There are a lot of people who could benefit.”
Leach said that because Dexcom can demonstrate the benefits of Stelo, the company believes insurance companies will eventually pay for it. He said Dexcom decided to bring the product to market at an “affordable” cash price first to help get it into users' hands quickly.
“I think it's important for people to have that perception: It's like a mirror on their body,” he said. “It's very personal.”
Don't miss these CNBC PRO stories: