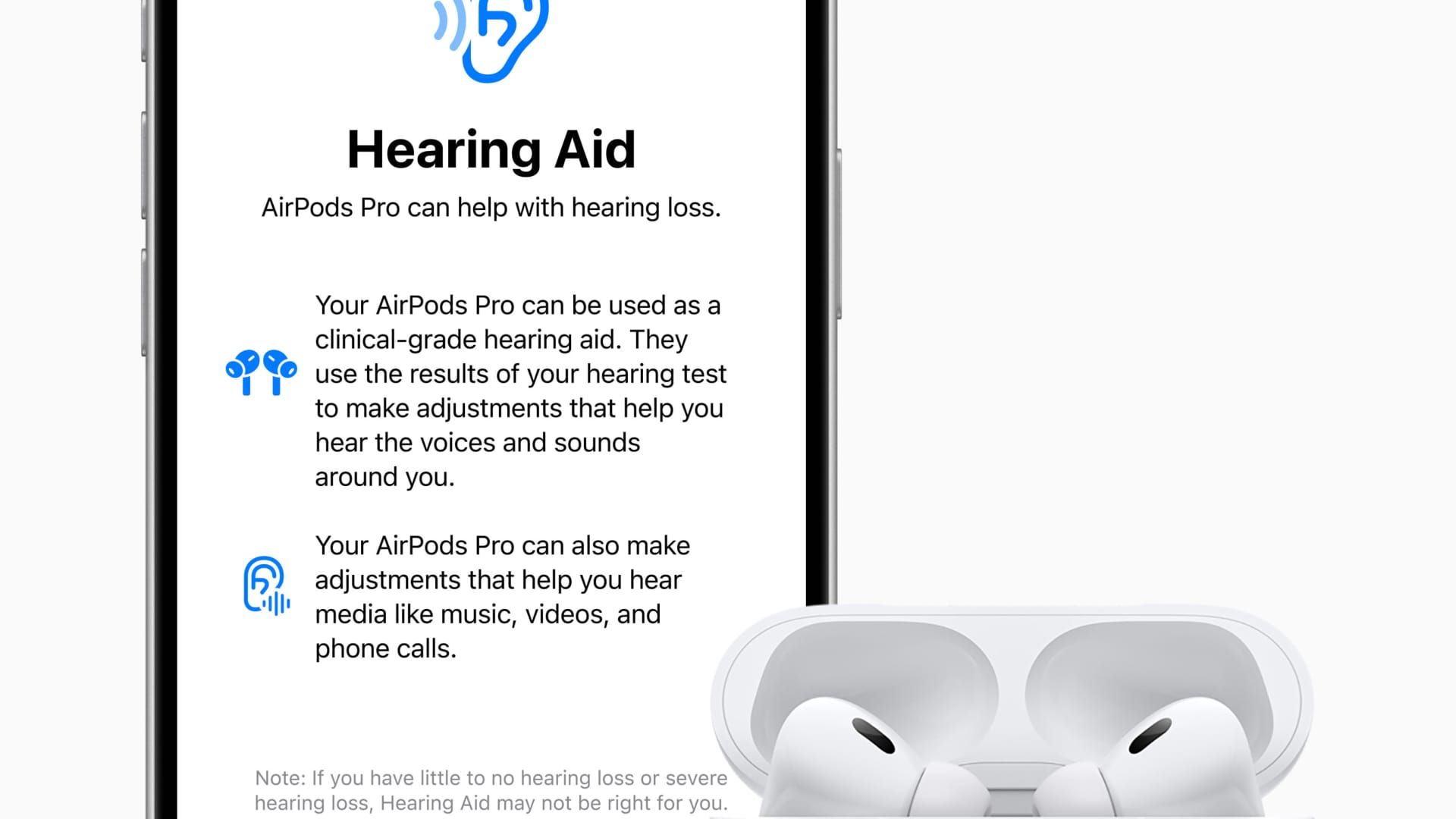
Soon, people wearing AirPods in their ears will no longer hear you, but will use them to hear you better.
Apple announced Monday that its AirPods Pro 2 earbuds will become an FDA-approved hearing aid in the coming weeks via a software update. That means adults with mild to moderate hearing loss — about 30 million Americans, according to the Food and Drug Administration — will be able to use Apple's earbuds to amplify specific sounds they want to hear better.
“After you take a hearing test, your AirPods Pro become a personalized hearing aid, boosting the specific sounds you need in real time, like parts of speech or elements of your environment,” Apple's vice president of health, Sumbul Desai, said in the feature's launch video.
The announcement is the latest example of Apple's push to enter the healthcare sector, a potential $15 trillion market by 2030, according to RBC Capital Markets. Apple CEO Tim Cook has highlighted health features as the company's “most important contribution to humanity.”
That strategy includes developing FDA-approved features for its wearables and replacing them with more expensive, purpose-built medical devices. Since 2020, Apple has added an irregular heartbeat notification service, an atrial fibrillation reader and an electrocardiogram reader to its Apple Watch, according to FDA filings.
The new feature is a free software update for select AirPods models and will be included with Apple's $249 AirPods Pro 2.
According to purchasing guides cited by the Hearing Loss Association of America, an advocacy group for the hearing impaired, many over-the-counter hearing aids are much more expensive. While some over-the-counter hearing aids cost as little as $99, most cost between $799 and thousands of dollars.
“What's really cool about Apple now saying that its AirPods can be over-the-counter hearing aids is that we're seeing that technological innovation at a price point and in a product that's very mainstream,” said Barbara Kelley, executive director of the Hearing Loss Association of America.
Apple is trying to revive AirPods sales after a few slow years.
The company doesn’t break out statistics for AirPods individually, but its wearables category declined 2% annually during the most recent quarter for which sales are available. Analysts say adding health features like a hearing aid expands the market for the device, which could help sales.
“The use of headphones is very niche,” said Gene Munster, founder of Deepwater Asset Management, who estimates that AirPods account for about 5% of Apple's total revenue. “This opens the door to a different market.”
How it works
The Apple Hearing Health experience requires a pair of Apple AirPods Pro headphones and an iPhone.
The company has built a hearing test into its devices within the Settings app. After the program checks that the earbuds fit properly in the user's ears, it plays a series of tones for about five minutes. The user has to tap the screen when they hear a tone.
This creates a profile of various frequencies and volume settings that the user may have trouble hearing, which are stored in the Health app. That profile can then be applied to turn AirPods Pro into custom hearing aids.
Apple said the test was scientifically valid and based on data it collected from its noise-detection apps and a study with 160,000 participants that began in 2019.
In a promotional video, Apple showed a mother putting on AirPods to better hear her son on his birthday.
At the counter
Apple's launch has been boosted by a recent regulatory change.
Previously, all hearing aids required a prescription after being examined by a certified audiologist. In 2022, the FDA opened the market to over-the-counter hearing aids that were significantly less expensive due to the use of audio testing software or at-home adjustments.
However, Apple's AirPods won't make other headphones obsolete right away.
Among its limitations is the battery, which lasts six hours, which is not enough for the type of all-day use that some over-the-counter hearing aids can do.
Additionally, AirPods Pro are only for people with mild or moderate hearing loss — that is, people who have trouble understanding speech in noisy environments. Anyone with “severe” or “profound” hearing loss still needs to see a licensed audiologist, experts said.
In addition, Apple's headphones still need FDA clearance.
Devices that use technology or software to customize the fit or settings of hearing aids require prior authorization from the agency, an FDA press official told CNBC. Apple is awaiting clearance from the FDA, as well as from regulators around the world, the company said Monday.
Bridget Dobyan, executive director of the Hearing Industries Association, said she welcomed Apple's entry into the market to raise awareness about hearing health, but there are still many hearing loss situations that require a medical approach.
“Over-the-counter hearing aids may be appropriate for adults with mild to moderate hearing loss, but consulting a licensed hearing professional can also help determine specific hearing health needs,” Dobyan said.
It's not uncommon for Apple's foray into healthcare to draw criticism from traditional players, who say the tech company's features are no substitute for actual medical devices.
For example, Joe Kiani, CEO of Masimo, a medical device company currently in litigation with Apple over intellectual property and business practices, said earlier this year that the Apple Watch's pulse oximeter feature was being “masqueraded” as “a reliable medical pulse oximeter.”
Following a legal victory over patents, Masimo forced Apple in January to turn off the pulse oximeter on newly sold Apple Watch devices.