Multiple sclerosis, an autoimmune disease that affects 2.9 million people, presents a biological conundrum.
Many researchers suspect that the disease is caused by a virus, known as Epstein-Barr, which causes the immune system to attack the nerves and can cause patients to have difficulty walking or talking. But the virus cannot be the whole story, since almost everyone is infected with it at some point in life.
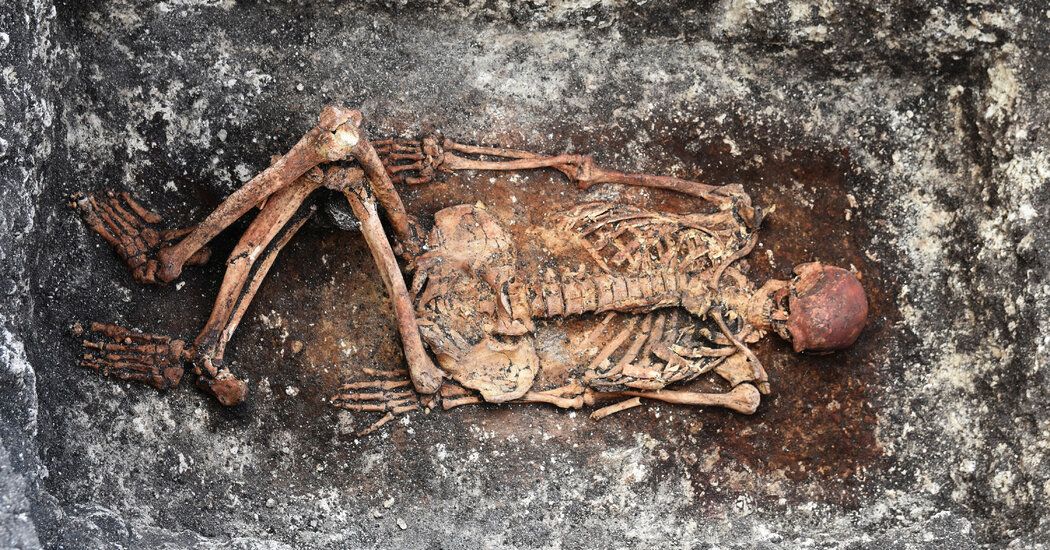
A new study has found a possible solution to this paradox in the skeletal remains of a lost tribe of nomads who herded cattle on the steppes of western Asia 5,000 years ago. It turns out that the nomads carried genetic mutations that likely protected them from pathogens carried by their animals, but also made their immune systems more sensitive. These genes, the study suggests, made the descendants of nomads prone to an uncontrolled immune response.
The finding is part of a broader, unprecedented effort to understand how the evolutionary past has shaped the health of living people. Researchers are analyzing thousands of genomes from people who lived between Portugal and Siberia and between Norway and Iran approximately 3,000 to 11,000 years ago. They hope to trace the genetic roots not only of multiple sclerosis, but also of diabetes, schizophrenia and many other modern diseases.
“We are taking ancient human genomics to a completely new level,” said Eske Willerslev, a geneticist at the University of Copenhagen who led the effort.
The researchers published the study on multiple sclerosis, as well as three other articles on the genetics and health of ancient people, in the journal Nature on Wednesday.
For more than a decade, Dr. Willerslev and other researchers have been extracting DNA from ancient human bones. By comparing surviving genetic material with that of living people, scientists have been able to track some of the most important migrations of people around the world.
For example, they have recorded the movement of farmers from what is now Turkey through Europe from about 8,000 years ago. These early farmers encountered European hunter-gatherers who had lived on the continent for more than 30,000 years. In some places, hunter-gatherer DNA disappeared from skeletons after farmers arrived, suggesting violent conflict. Elsewhere, the two populations mixed enough to produce later generations with mixed ancestry.
Thousands of years passed before the next great migratory shift. About 5,000 years ago, European DNA began to show the genetic signatures of a group of shepherds living in the steppes stretching from Ukraine to Kazakhstan, called the Yamnaya.
The Yamnaya traveled by horse and cart across hundreds of miles of grasslands, herding cows, goats, and sheep along the way. Even without farms or cities, they prospered for centuries, burying their dead with gold and jewels.
In the Bronze Age, the Yamnaya expanded their territory, devastating much of Asia and Europe. Dr. Willerslev and his colleagues discovered that once in Europe, the group often eliminated farmers they encountered, although they also maintained peaceful relations in some places.
Today, the people of northern Europe can trace most of their ancestry to the Yamnaya. Further south, Yamnaya ancestry is less common. Instead, the people there have more ancestry from Near Eastern farmers and early European hunter-gatherers.
Dr. Willerslev and his colleagues wondered what kind of genetic variations each ancient group carried and how they affected their health. To find out, researchers studied some of their living descendants.
They tapped into the UK Biobank, a huge database of DNA and medical information. Most of the 433,395 volunteers the scientists studied were born in Britain, but 24,511 were born in other countries.
The researchers were able to link thousands of genetic variants in the database to increased risks of a wide range of diseases. They then compared the volunteers' DNA with genetic fragments from ancient skeletons.
One analysis found that Western European hunter-gatherers, for example, carried many of the variants that increase the risk of high cholesterol, high blood pressure and diabetes. Another showed that ancient Near Eastern farmers had a high burden of variants linked to anxiety and other mood disorders.
These findings do not necessarily mean that these ancient people suffered from these conditions. Genetic variants set the trap, but it is often the environment that sets it off.
Diabetes, for example, has become increasingly common in the modern world, in part due to the cheap, sugar-laden foods that make up an increasing part of our diet. In previous centuries, high-risk genes for diabetes may not have had the opportunity to lead to the disease.
In some cases, Dr. Willerslev and his colleagues found, these genetic variants provided ancient people with a survival advantage.
Variants that increase the risk of multiple sclerosis, for example, became increasingly common among the Yamnaya. The nomads who wore them seem to have had more offspring than those who did not wear them.
“These variants that cause the high risk of multiple sclerosis today must have had a benefit in the past,” Dr. Willerslev said.
The new studies give some solid clues about what that benefit is. Some of the skeletons contained DNA not only from humans, but also from disease-causing viruses and bacteria. Many of these pathogens did not appear among hunter-gatherers or even early farmers in Europe. But Yamnaya's remains contained genetic signatures of several pathogens, including the one that caused the plague.
“These variants appear to provide some protection against infectious diseases,” Dr. Willerslev said.
Several studies on multiple sclerosis suggest that variants that increase the risk of the disease also make the immune system's attack against viruses and bacteria more aggressive.
Dr. Willerslev and his colleagues argued that the Yamnaya were more vulnerable to animal diseases than earlier humans. The Yamnaya depended on animals for meat and milk and were in constant contact with their herds as they moved across the steppes.
Those conditions provided a new opportunity for diseases to jump to humans. In response, the Yamnaya developed genes related to the immune system that helped them defend themselves against new enemies.
“They've made a really compelling case,” Yassine Souilmi, a genomicist at the University of Adelaide in Australia, said of Dr. Willerslev and his colleagues. “I would be surprised if more experimental evidence does not agree with their conclusions.”
Dr. Lars Fugger, a multiple sclerosis expert at the University of Oxford who collaborated with Dr. Willerslev on the new studies, said the disease may not have become common until recent decades. In today's environment, with fewer infectious diseases than in past centuries, he said, a strong immune system is more likely to fail and attack its own body.
“Many of us live in a squeaky clean environment,” Dr. Fugger said. “The balance is no longer there.”
Understanding the evolutionary roots of multiple sclerosis could guide researchers toward better treatments for the disease. Currently, the only effective treatments for the condition are drugs that suppress the immune system. To Dr. Fugger, such drugs seem like blunt instruments against a delicately balanced part of our biology.
“Instead of just removing it, we should try to figure out in more detail how it is out of balance and then try to recalibrate it,” he said.
Researchers are beginning similar analyzes of other diseases, such as schizophrenia and psoriasis. “This is just the beginning,” said Dr. Fugger.
For now, they continue to rely on the UK Biobank, meaning their results will be largely limited to genes that have influenced the health of northern Europeans. “It would be phenomenal to have similar studies in other parts of the world,” said Lluís Quintana-Murci, an evolutionary geneticist at the Pasteur Institute who was not involved in the research.
But there are few opportunities to conduct such studies. Many countries lack detailed electronic medical records, for one thing. And the unethical behavior of Western scientists has left many indigenous populations uninterested in donating DNA for such efforts.
Dr Souilmi, who is helping to create a database for indigenous Australians, said the different evolutionary path of each population could reveal important insights into human biology in general. “By studying other parts of the world, we are actually expanding our understanding of all current human conditions,” he said.