Good afternoon! The launch of the closely watched Alzheimer's drug Leqembi is off to a slow start.
Even so, the demand for treatment by biogen and his Japanese partner Eisai is increasing.
That's the update the two drugmakers gave Wall Street during their latest quarterly earnings calls earlier this month.
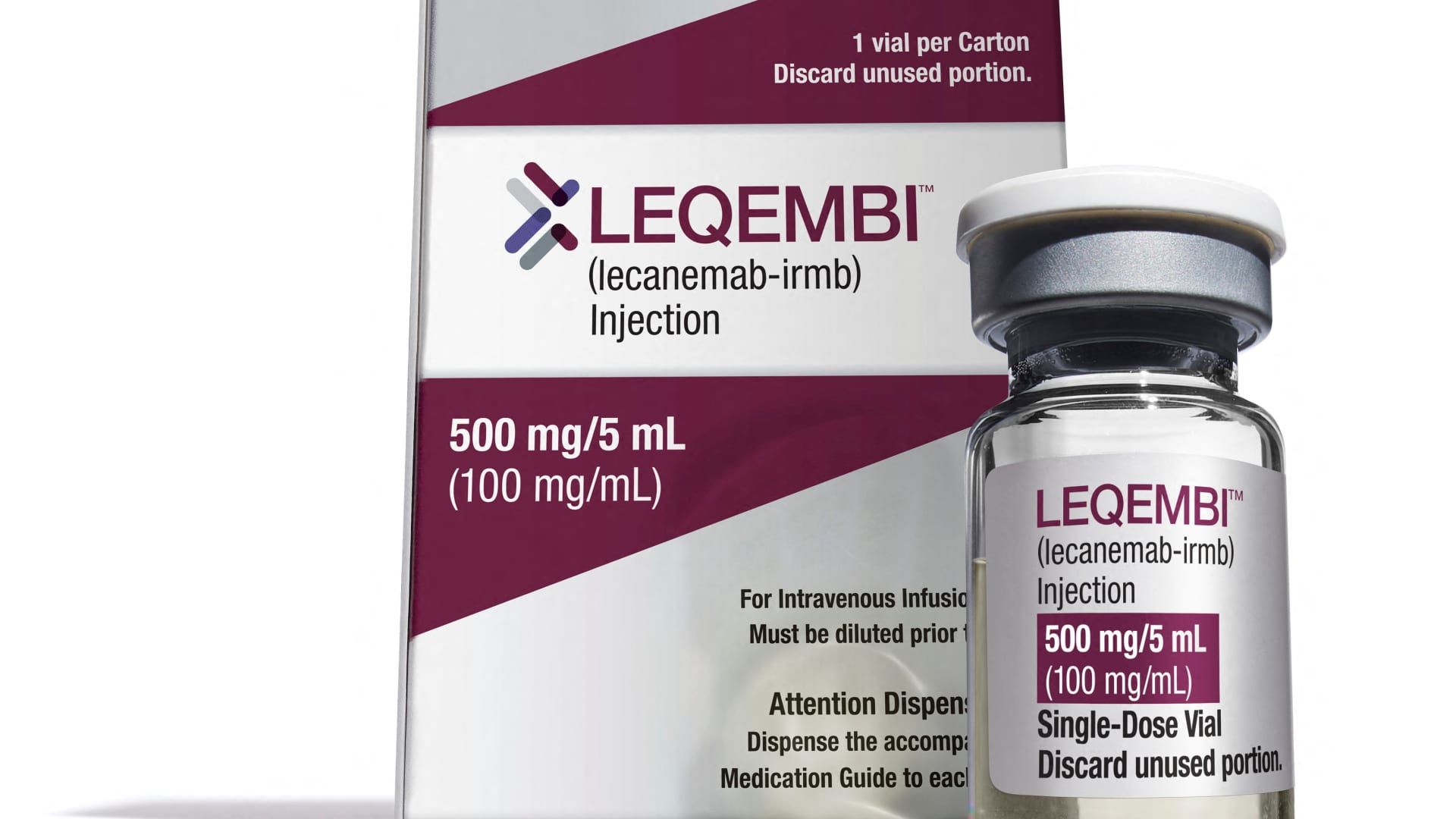
But before we get into the numbers, here's why the treatment is so important: Leqembi became the first drug that slows the progression of Alzheimer's disease to gain approval in the U.S. when the Food and Drug Administration approved it. authorized in July.
While the therapy is not a cure, its implementation was a historic moment for the more than 6 million older Americans who suffer from this difficult-to-treat condition.
Leqembi is also critical to the future of Biogen's business.
The company is pinning its hopes on the drug to drive growth as it cuts costs and sees sales of its multiple sclerosis therapies decline, some of which face generic competition. Leqembi also marks a promising new chapter for Biogen's Alzheimer's portfolio after the polarized approval and 2021 launch of an older drug, Aduhelm, which the company abandoned last month.
Only about 2,000 patients are currently taking Leqembi, seven months after it gained FDA approval, Biogen CEO Chris Viehbacher told reporters on a media call last week. That makes Biogen's goal of 10,000 patients treated by the end of March look increasingly elusive.
But Viehbacher said the company expected a “progressive ramp” and is seeing considerable demand for the drug.
Biogen estimates that 3,800 patients have enrolled in multiple registries to receive Leqembi as of last month, meaning they have been prescribed the drug or are about to receive it, according to Viehbacher. She said that suggests between 260 and 265 patients were being added to registries each week starting in January, about a 56% increase since December.
Morgan Stanley analysts called the registry data “encouraging” in a note last week, but said the firm is waiting for those patients to begin therapy.
Viehbacher maintains that demand is not the problem. The real hurdle is having systems that can “adapt to this new flow of patients.”
Viehbacher is referring to the complex process patients must go through before receiving Leqembi. To obtain the medication, the patient often needs to make an appointment with a neurologist, which can be a difficult task on its own.
The neurologist must then perform special tests to confirm that the patient is in an early stage of Alzheimer's disease and has a sticky, toxic protein called beta-amyloid, which is a hallmark of the disease. If eligible, the patient will be prescribed Leqembi, which is administered as a one-hour infusion every two weeks.
Viehbacher also highlighted challenges related to MRI monitoring during a separate earnings conference call with investors. Leqembi's prescribing label says patients should undergo multiple MRIs during the first year of treatment to look for signs of ARIA, a side effect that causes swelling or bleeding of the brain and can be fatal in rare cases.
But “when we start the infusion, you have to have the first MRI within the first two weeks,” Viehbacher said during the call. Therefore, some patients don't want to start taking the medication until their first MRI appointment is scheduled, she said.
“Until people understand this, get all that coordination, I think that's where it seems to be…one of the bottlenecks,” Viehbacher said.
Leqembi generated $7 million in fourth-quarter revenue and $10 million in full-year sales, according to Eisai. Wall Street analysts expect much more this year.
“Growth is good, but we need to see a big acceleration,” Jefferies analysts wrote in a note last week.
Notably, Biogen and Eisai are also working to get the FDA to approve an injectable version of Leqembi, which showed promising initial results in a clinical trial in October.
The injectable form would be a new, more convenient option for delivering antibody treatment to patients, potentially paving the way for wider acceptance.
And expanded use can't come soon enough for drugmakers: Eli Lilly's Alzheimer's drug, donanemab, could win FDA approval any time this quarter.
The latest in health technology
The headphones make some users nauseous. Here's what experts say can help
Apple's Vision Pro headphones are displayed at the Apple Fifth Avenue store in Manhattan, New York, U.S., February 2, 2024. REUTERS/Brendan McDermid
Brendan Mcdermid | Reuters
It's been a few weeks since Apple launched its new mixed reality headset, the Vision Pro, after months of anticipation. CEO Tim Cook told CNBC's Jim Cramer that headphones are “the technology of tomorrow today,” and CNBC's Todd Haselton called it the “most fun” new product he's tried in years.
But headphones are not for everyone. Some early users said they experienced discomforts such as headaches and dizziness.
For example, in a post on social media site X, one user said Apple's Vision Pro caused severe dizziness, “just like any other virtual reality.” Another person experienced nausea less than 30 minutes after using the device and urged others to “proceed with caution” if they get dizzy frequently. Still, this person called the Vision Pro a “truly amazing experience,” according to an X post.
Apple published a list of tips to help users if they experience motion sickness while using Vision Pro, such as taking a break, reducing window size, and avoiding experiences that involve a lot of motion. The company said users should talk to their doctor if their symptoms are severe and persistent, according to the site.
Feelings of nausea and headaches are not unique to the Apple device, and users have encountered these problems with headphones from companies like Meta for years. CNBC spoke to some experts to understand why some people have trouble using virtual reality, augmented and mixed reality headsets.
Dr. Joanna Jen, a neurologist at Mount Sinai Health System, said much is still unknown about dizziness, although there are some major theories about why it occurs. She said a popular theory is that dizziness occurs when there is an incompatibility or mismatch between the sensory inputs the brain and body receive.
For example, if you are flying with headphones but are relatively still, that would be a sensory mismatch.
Jen said that everyone has a different sensitivity and that some people can adapt and develop a tolerance to headphones over time. She said if you feel like you need to take a break, do so and you can always try again later.
“Moderation is key,” Jen said in an interview. “Don't overdo it.”
Dr. Natascha Tuznik, an infectious disease specialist and associate professor at the University of California, Davis, helps run the school's travel clinic, where dizziness crops up frequently. Specifically with headsets, he said users can try to alleviate symptoms by reducing the length of their sessions, making sure the device is installed and set up correctly, moving around by carrying or bending them inside the headset instead of walking when appropriate, and looking for games that have a static reference point.
Tuznik said there's no good way to predict whether you'll experience motion sickness, so it might be worth trying a pair of headphones at a friend's house or in a store.
“If you know how you react to non-VR situations where you might get dizzy, for example how you react in a car, on a ride, or on a boat, then there's a good chance you'll experience a similar situation.” degree of effects when using virtual reality,” Tuznik told CNBC.
In essence, listen to your body! Take note of how you feel when wearing an immersive headset and don't force it if it doesn't feel right. Unfortunately, some technologies just don't work for everyone. The bright side, however, is that nausea could save you $3,500 at the Apple Store.
Feel free to send tips, suggestions, story ideas, and facts to Annika at [email protected] and Ashley at [email protected].