Drug makers are betting that delivering radiation directly to tumors will become the next big cancer breakthrough.
Bristol-Myers Squibb, AstraZeneca, Eli Lilly and other pharmaceutical companies have spent about $10 billion on deals to acquire or work with radiopharmaceutical makers. They have bought smaller startups to get their hands on technology that, although in its early stages, could treat numerous types of cancer.
“Any large company that has a commercial presence in oncology or for whom oncology is an important therapeutic category will likely need exposure in this area in one form or another,” said Guggenheim Securities analyst Michael Schmidt.
According to Schmidt, there are already two Novartis radiopharmaceuticals available and dozens more in development. It is difficult to estimate the total market opportunity because there are so many types of cancer that could be treated with these drugs, he said.
Schmidt predicts the category could grow to at least $5 billion in revenue if the technology is limited to treating a few types of cancer, such as prostate and neuroendocrine tumors. Up to tens of billions if proven to be true Effective in more cancers.
The drugs work by attaching radioactive material to a specific molecule that seeks out and attaches to a specific marker on cancer cells. The trick is finding markers that exist in cancer cells but not in healthy cells. This may allow the treatment to target radiation to the cancer cells and spare the rest of the body from the same level of radiation. damage that comes with many cancer drugs.
It took time to prove that the technology could work both scientifically and economically. The first radiopharmaceuticals were approved in the early 2000s, but interest from big pharmaceutical companies only increased recently.
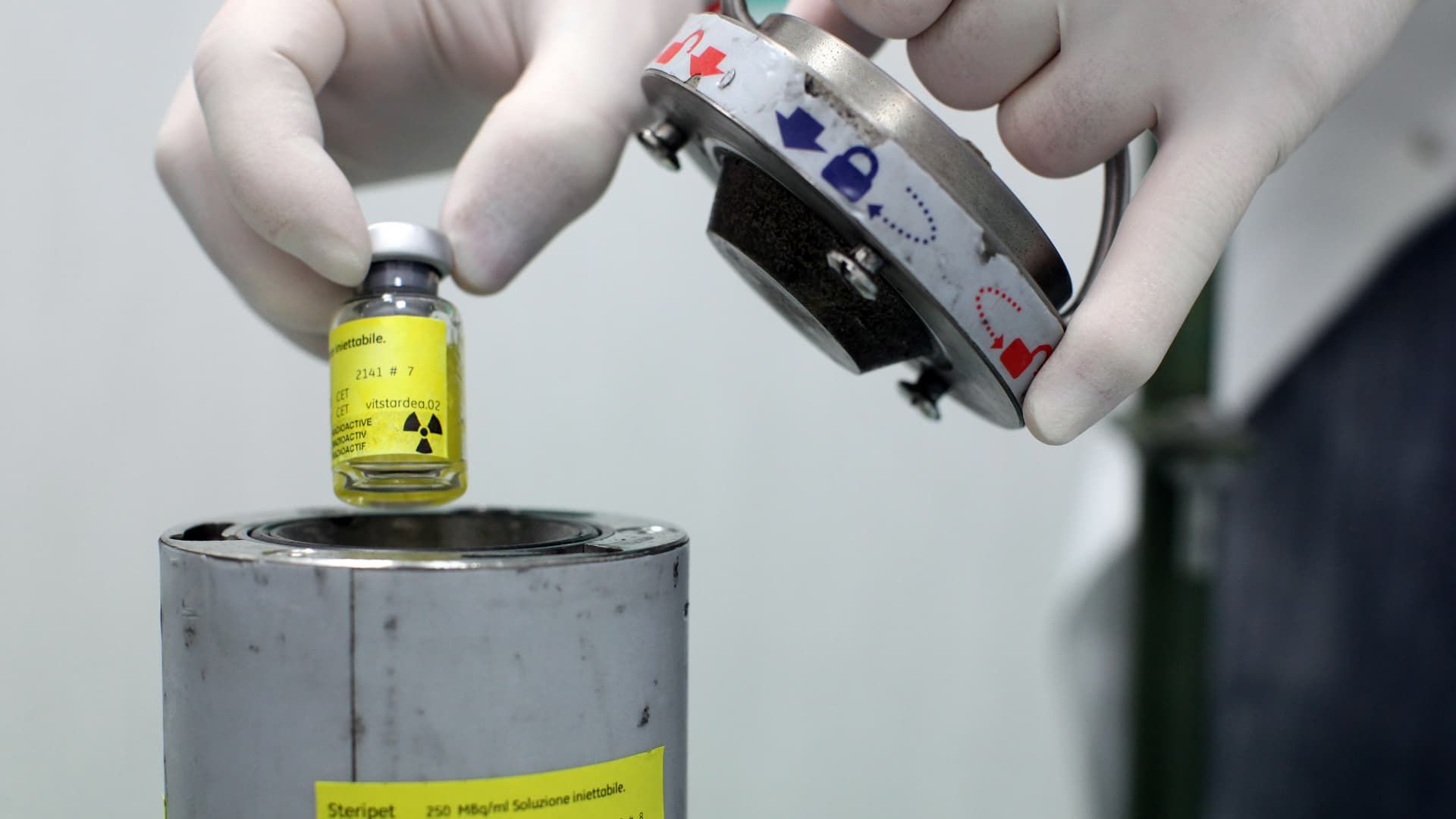
An employee works at the NSA radiopharmaceutical plant in Aedea, Rome, Italy.
Franco Origlia | Getty Images
Manufacturing the drugs requires complex logistics and manufacturing processes, two major drawbacks. The radioactive material degrades quickly, so patients must be treated within days of receiving the treatment.
Pharmaceutical companies demonstrated that they could handle complex and time-sensitive drugs such as CAR-T therapies for blood cancers or genetic therapies for rare diseases. Then Novartis showed that these strategies could be applied to radiopharmaceuticals.
The Swiss pharmaceutical giant won approval in 2018 for a radiopharmaceutical called Lutathera for a rare type of cancer in the pancreas and gastrointestinal tract. In 2022, Novartis won another approval for its prostate cancer treatment Pluvicto. Together, the drugs are expected to reach about $4 billion in sales by 2027, according to FactSet consensus estimates.
These successes sparked broader interest in radiopharmaceuticals.
“We took all of that together and thought we should do something, we need to close deals here,” said Jacob Van Naarden, president of Eli Lilly's oncology business.
Last year, Lilly acquired radiopharmaceutical maker Point Biopharma for about $1.4 billion and also signed some partnerships with companies developing the treatments. One of the most important factors during Lilly’s initial search was whether the companies were prepared to make the drugs, Van Naarden said. Radiopharmaceuticals are not easy to make, and Lilly wanted to make sure any initial acquisition could produce the drugs itself rather than outsourcing the work.
Manufacturing was also a key component of Bristol Myers Squibb's $4.1 billion acquisition of RayzeBio, said Ben Hickey, RayzeBio's president. At the time of the acquisition, RayzeBio was nearing completion of a manufacturing facility in Indiana and had secured its own supply of radioactive material needed to develop the experimental drugs in its pipeline.
“It was clearly one of the criteria to make sure we had our destiny in our hands,” Hickey said.
Novartis has shown why this is so important, as the company struggled to produce sufficient doses of Pluvicto. It is investing more than $300 million to open and expand radiopharmaceutical manufacturing plants in the United States so it can produce the drug and get it to patients quickly. The company can now meet demand for the treatment, which involves careful planning for its distribution.
According to Victor Bulto, president of Novartis’ U.S. division, each dose carries a GPS tracker to ensure it reaches the right patient at the right time. Novartis transports the doses to destinations within nine hours of the factory to minimize the risk of disruptions from storms, Bulto said.
The complexity is also felt by doctors and patients receiving treatment.
Bassett Healthcare Network in upstate New York needed to update its medical license to handle radioactive material before administering Lutathera and Pluvicto, said Dr. Timothy Korytko, Bassett’s chief radiation oncologist. A board-certified specialist must administer the drugs, which are given intravenously.
It can take several weeks from the time a radiopharmaceutical is prescribed until it is administered. In the case of Pluvicto, patients come once every six weeks for up to six treatments.
Radiopharmaceuticals begin to decompose once they are manufactured, so they are only useful for a few days.
Ronald Coy and his wife Sharon.
Courtesy: Ronald Coy
Ronald Coy knows how important it is to keep his appointments. Coy, a retired firefighter who has been battling prostate cancer since 2015, drives more than an hour across upstate New York to receive Pluvicto in Bassett. Coy hasn't had any problems so far, but he's worried a snowstorm could ruin one of his appointments between now and the end of January.
“We hope there won't be any major storms until then, or if there are, it will be a week before I leave,” Coy said.
When Coy returns home after treatment, he must take precautions, such as staying away from his wife Sharon so she doesn't get exposed to radiation. He drinks plenty of water to flush the extra radiation out of his body. He doesn't mind minor inconveniences for a few days if it means fighting cancer.
For Novartis, investing in the infrastructure to produce and distribute radiopharmaceuticals would be worthwhile for Pluvicto and Lutathera alone, Bulto said. But it is even more attractive because of the potential to treat more cancers. He cites as an example Novartis' work to develop a drug for a cancer marker found in 28 different tumors, including breast, lung and pancreatic cancer.
“If we could put all this knowledge we have developed from product distribution to the service of patients with lung and breast cancer, and potentially demonstrate these significant levels of efficacy and tolerability, we would be talking about a very large potential impact on cancer treatment and, of course, also a very viable business,” he said.
At this point, it's still an open question. The field is in its infancy, executives say, and the promise of radiopharmaceuticals beyond the current cancers they treat remains to be proven.
“If we can be successful in expanding the repertoire of targets and tumor types, this could be a very large class of drugs,” Eli Lilly's Van Naarden said, adding that at this point it's hard to say whether the class will be “super important” or “just important.”
One opportunity Bristol Myers Squibb sees is combining radiopharmaceuticals with existing cancer drugs, such as immunotherapy, said Robert Plenge, Bristol's chief research officer. AstraZeneca shares that view.
AstraZeneca spent $2 billion to acquire Fusion Pharmaceuticals earlier this year. Susan Galbraith, the company's executive vice president of oncology research and development, points to existing treatments that combine immunotherapy with radiation therapy.
Galbraith said the size of AstraZeneca's radiopharmaceuticals portfolio will ultimately depend on its initial prostate cancer program and other undisclosed targets already underway. But he believes the technology will become an important part of cancer medicines in the next decade.
It could take years to fully understand the technology's potential, as many experimental drugs are still in the early stages of development. One remaining question is whether other radiopharmaceuticals are as safe and well tolerated as Novartis' Pluvicto, especially those that use other types of radioactive material, Guggenheim analyst Schmidt said.
Ronald Coy has been battling prostate cancer for nearly 10 years. He started taking Novartis' Pluvicto earlier this year.
Courtesy: Ronald Coy
Big pharmaceutical companies are not waiting to join the race. Stories like Coy's encourage them to believe that the work will pay off.
For nearly 10 years, Coy has undergone multiple treatments for prostate cancer that has spread to his bones. After a single treatment with Pluvicto earlier this year, blood tests showed Coy's cancer level plummeted.
Not everyone responds so well to Pluvicto, and things can always change for Coy. But for now, Coy feels lucky to be among the group that responds well to Pluvicto. That's worth the travel and precautions he has to take.
“I feel very fortunate every day to be, as things stand now, part of the third team where this is working really well for me,” he said.
— CNBC Leanne Miller contributed to this report.