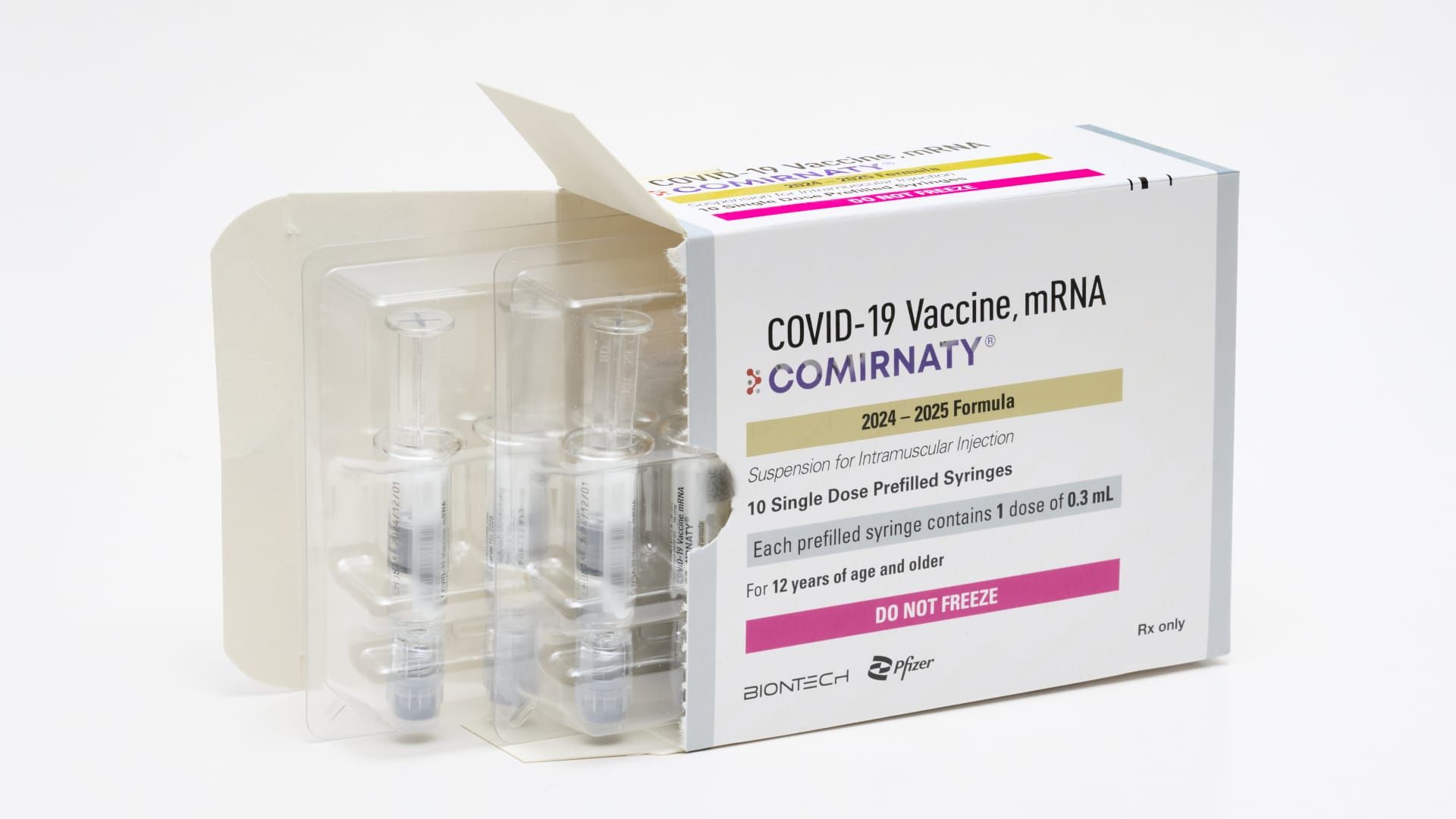
The Food and Drug Administration on Thursday Updated Covid vaccines approved Pfizer and Modernputting the new vaccines on track to reach most Americans in the coming days amid a summer surge in the virus.
The vaccines target a strain called KP.2, a descendant of the highly contagious JN.1 omicron subvariant that began circulating widely in the United States earlier this year. KP.2 was the dominant Covid strain in May but now accounts for only about 3% of all cases in the United States as of Saturday, according to the latest data from the Centers for Disease Control and Prevention.
Still, Pfizer and Moderna have said their KP.2 vaccines may produce stronger immune responses against other circulating JN.1 subvariants, such as KP.3 and LB.1, than last year's round of shots targeting the omicron XBB.1.5 strain..
“Given waning population immunity from prior exposure to the virus and prior vaccination, we strongly encourage those who are eligible to consider receiving an updated COVID-19 vaccine to provide improved protection against currently circulating variants,” Dr. Peter Marks, director of the FDA's Center for Biologics Evaluation and Research, said in a statement.
In June, the CDC recommended that everyone 6 months and older get an updated COVID vaccine and flu vaccine this year. The new Pfizer and Moderna vaccines are specifically approved for people 12 years and older and are authorized under emergency use for children ages 6 months to 11 years.
Pfizer will begin distributing its new vaccine immediately and expects it to be available in pharmacies, hospitals and clinics across the United States “starting in the coming days,” the company said in a statement. Moderna expects its vaccine to be available in the coming days, according to a statement.
“Staying up to date with your COVID-19 vaccine remains one of the best ways to protect yourself and prevent serious illness,” Moderna CEO Stephane Bancel said in a statement. “We appreciate the U.S. FDA’s timely review and encourage people to talk to their health care providers about receiving their updated COVID-19 vaccine along with their flu vaccine this fall.”
The FDA approval comes just weeks before last year's round of vaccines, which the agency authorized on Sept. 11.
The early arrival of updated vaccines could offer some reassurance to Americans as the country experiences a relatively large surge of the virus this summer. A “high” or “very high” level of Covid is being detected in wastewater in nearly every state, according to CDC data. Wastewater monitoring provides insight into how widespread the virus is in the U.S. as other forms of testing have declined.
Other indicators of the virus are increasing but remain well below levels reached at the peak of the pandemic. COVID-19 test positivity rates rose to 18.3% in the week ending Aug. 10, up from 17.9% the week before, according to the CDC.
Meanwhile, the CDC said about 4 people are hospitalized for Covid per 100,000 people in a given area. That's up from about 1 Covid hospitalization per 100,000 people in May, which was the lowest level since the pandemic began.
The summer wave of COVID-19 could subside when vaccines reach patients and generate an immune response against the virus, which typically occurs two weeks after vaccination.
Still, federal health officials have long told Americans to expect annual updates to Covid-19 vaccines as the virus produces new strains that can bypass the immunity people have from previous vaccinations or infections — protection that fades over time. It’s similar to how the U.S. rolls out new flu vaccines each year.
It's unclear how many Americans will actually dare to get vaccinated again in the coming months.
Only about 22.5% of American adults received the latest round of vaccines that rolled out last fall, according to CDC data through early May.
According to a November survey by health policy research organization KFF, many Americans who received earlier doses of the Covid-19 vaccine cited a lack of concern about the virus as a reason for not getting the latest booster shot. Others said they had been too busy to get the vaccine, according to the survey.
In June, the FDA asked vaccine makers to make shots against JN.1 before telling them to target KP.2 instead “if possible.”
That change seemed to put Novavaxwhich applied for authorization for a new JN.1 vaccine that same month, is at a disadvantage. The FDA has not approved the biotech company's vaccine.
In a statement, Novavax said it is working “productively” with the FDA as the agency completes its review. Novavax expects its vaccine to receive authorization in time for the peak vaccination season in the United States.
The company said its vaccine provides protection against JN.1 descendants, including KP.2.3, KP.3, KP.3.1.1 and LB.1.
Novavax makes protein-based vaccines, which cannot be quickly updated to target another strain of the virus. Protein-based technology is a method that has been used for decades in routine vaccinations against hepatitis B and shingles.
Meanwhile, the Pfizer and Moderna vaccines use messenger RNA technology, which teaches cells how to make proteins that trigger an immune response against Covid. mRNA vaccines are much easier to develop and update than protein vaccines.